Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in this net ionic equation. HPO, CIO H,PO, HCIO Brønsted-Lowry Brønsted-Lowry Brønsted-Lowry Brønsted-Lowry In this reaction: The formula for the conjugate of CIO is The formula for the conjugate of H2PO, is
Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in this net ionic equation. HPO, CIO H,PO, HCIO Brønsted-Lowry Brønsted-Lowry Brønsted-Lowry Brønsted-Lowry In this reaction: The formula for the conjugate of CIO is The formula for the conjugate of H2PO, is
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter17: Acid-base(proton Transfer) Reactions
Section: Chapter Questions
Problem 3CLE
Related questions
Question
I don’t understand this question
Transcribed Image Text:Home
OWLV2 | Online tea X
O New Tab
/takeCovalentActivity.do?locator%=Dassignment-take
MyLab & Mastering..
Cengage Learning
Home | Navigate m HBO Max
» E
[References)
Use the References to access important values if needed for this question.
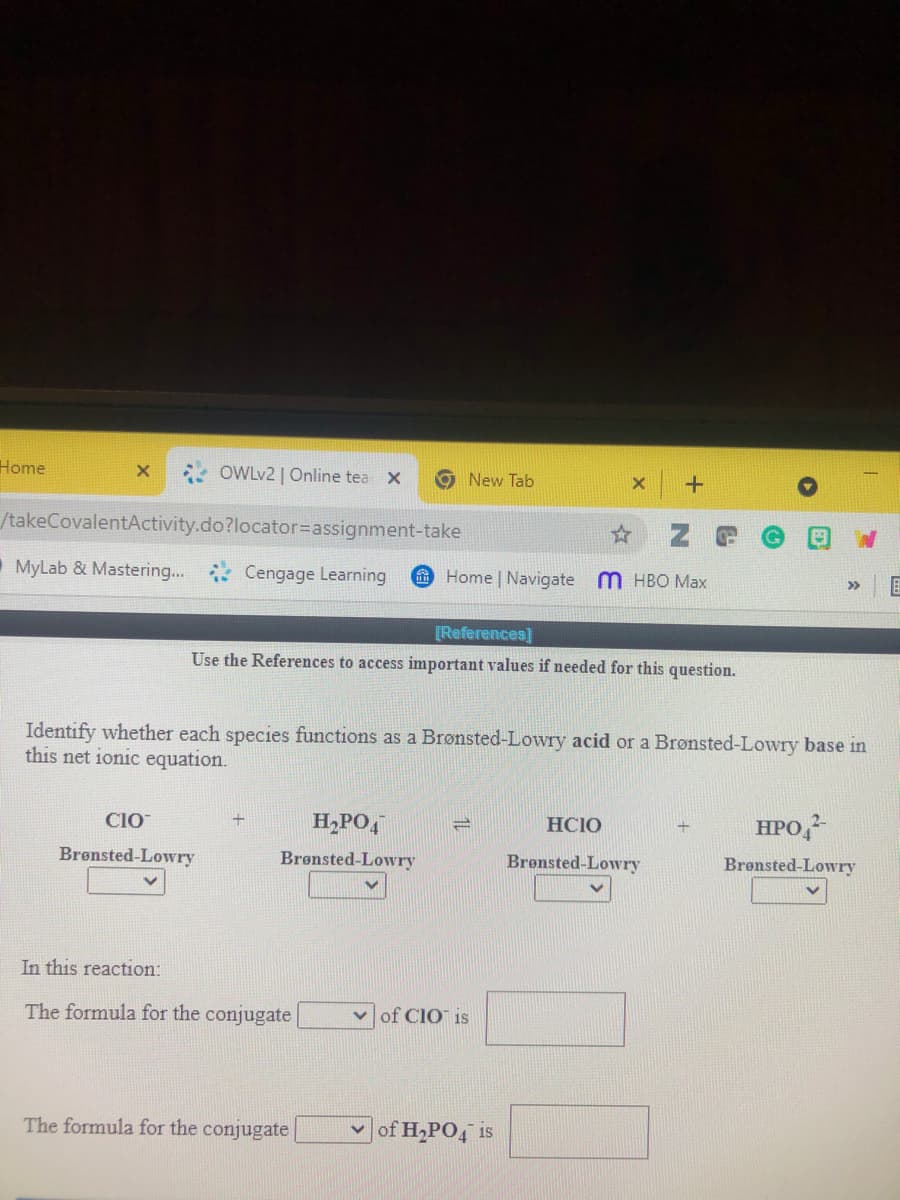
Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in
this net ionic equation.
H,PO,
HCIO
HPO,
CIO
Brønsted-Lowry
Brønsted-Lowry
Brønsted-Lowry
Brønsted-Lowry
In this reaction:
The formula for the conjugate
of CIO is
The formula for the conjugate
|of H,PO, is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning