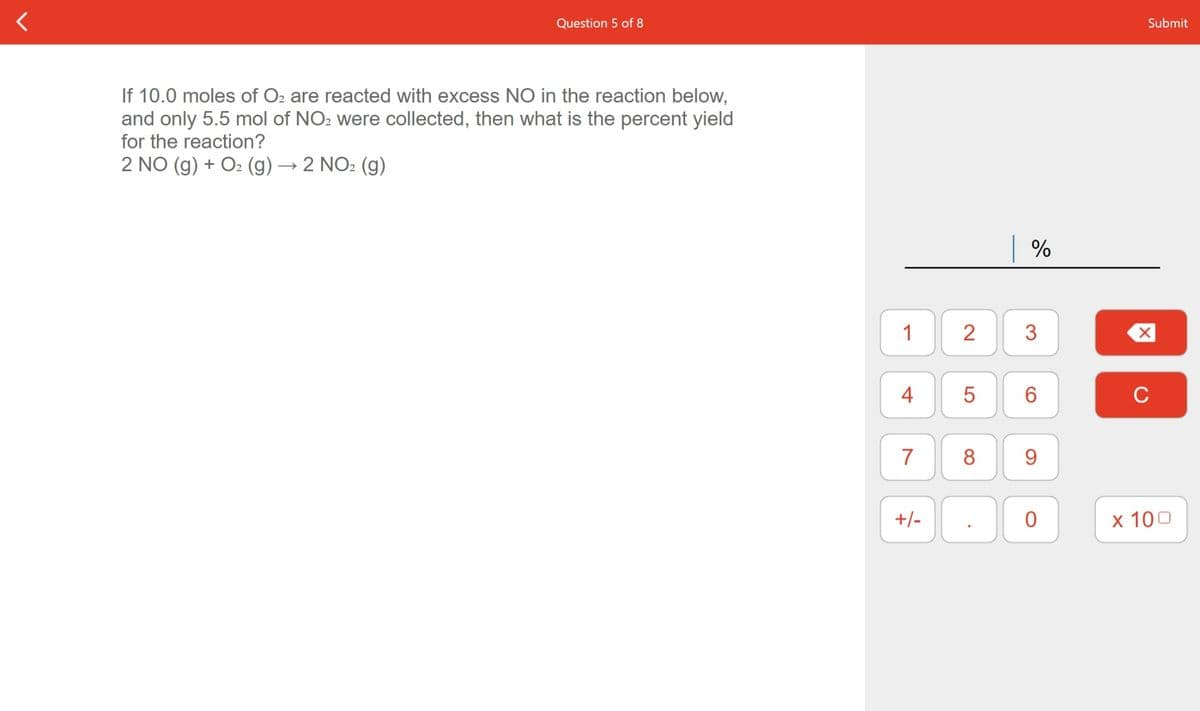
If 10.0 moles of O: are reacted with excess NO in the reaction below, and only 5.5 mol of NO2 were collected, then what is the percent yield for the reaction? 2 NO (g) + O2 (g)→2 NO2 (g)
If 10.0 moles of O: are reacted with excess NO in the reaction below, and only 5.5 mol of NO2 were collected, then what is the percent yield for the reaction? 2 NO (g) + O2 (g)→2 NO2 (g)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 71AP: When elemental copper is strongly heated with sulfur, a mixture of CuS and Cu2Sis produced. with CuS...
Related questions
Question
Transcribed Image Text:Question 5 of 8
Submit
If 10.0 moles of O2 are reacted with excess NO in the reaction below,
and only 5.5 mol of NO2 were collected, then what is the percent yield
for the reaction?
2 NO (g) + O: (g) → 2 NO2 (g)
%
1
2
3
4
5
C
7
8
9.
+/-
x 100
ㅇ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning