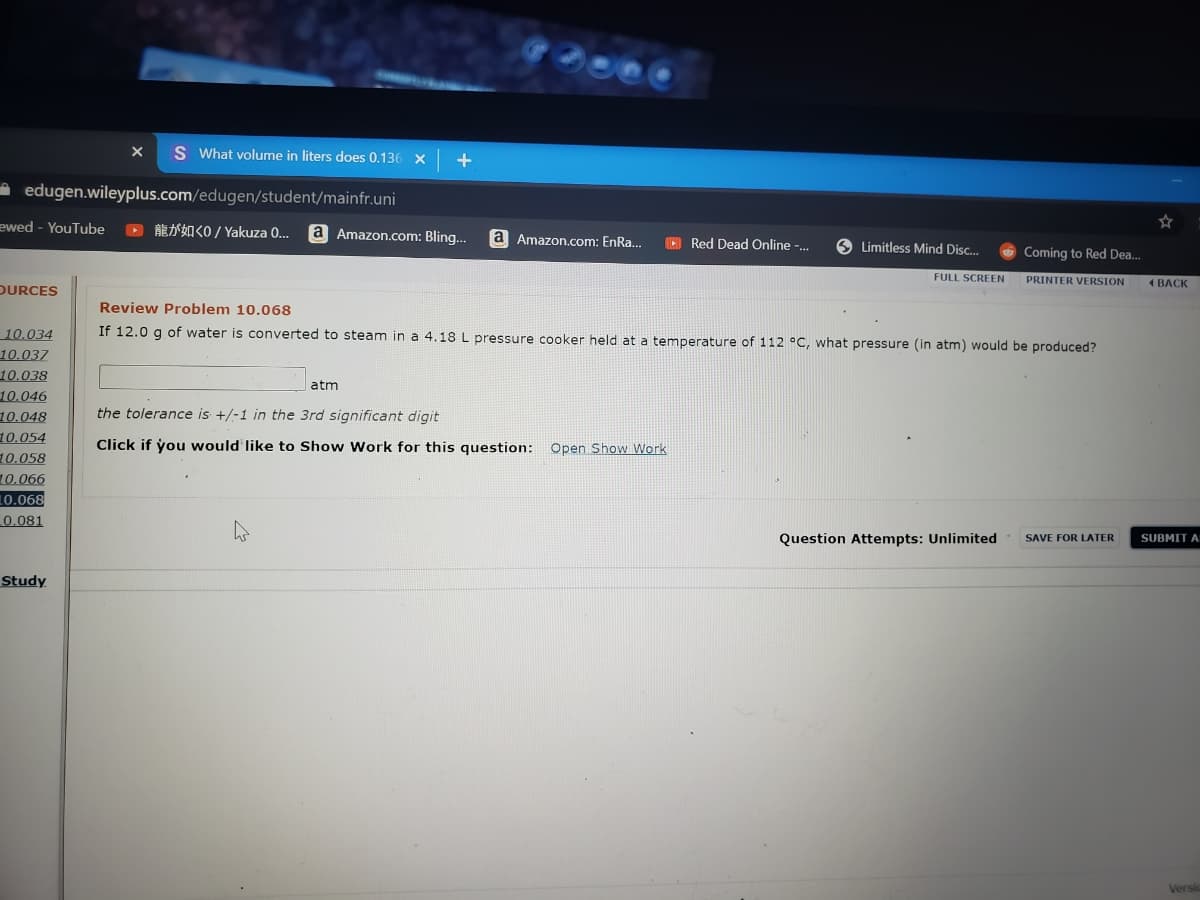
If 12.0 g of water is converted to steam in a 4.18 L pressure cooker held at a temperature of 112 °C, what pressure (in atm) would be produced?
If 12.0 g of water is converted to steam in a 4.18 L pressure cooker held at a temperature of 112 °C, what pressure (in atm) would be produced?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.107PAE: 107 A soft drink can’s label indicates that the volume of the soda it contains is 12 oz or 355 mL....
Related questions
Question
100%
Please help me with this HW question. Thank you! If you could show work so I could figure it out that would be great!
Transcribed Image Text:S What volume in liters does 0.136 X
A edugen.wileyplus.com/edugen/student/mainfr.uni
ewed - YouTube
O AEDA<O / Yakuza 0..
a Amazon.com: Bling..
a Amazon.com: EnRa.
O Red Dead Online -.
Limitless Mind Disc.
Coming to Red Dea.
FULL SCREEN
PRINTER VERSION
1 BACK
DURCES
Review Problem 10.068
10.034
If 12.0 g of water is converted to steam in a 4.18 L pressure cooker held at a temperature of 112 °C, what pressure (in atm) would be produced?
10.037
10.038
10.046
10.048
atm
the tolerance is +/-1 in the 3rd significant digit
10.054
10.058
Click if you would like to Show Work for this question:
Open Show Work
10.066
10.068
0.081
Question Attempts: Unlimited
SAVE FOR LATER
SUBMIT A
Study
Versic
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning