Water was drained from a buret between the 0.12- and 15.78-mL marks. The apparent volume was 15.78 - 0.12 = 15.66 mL. Measured in air at 25 o C, the mass of water delivered was 15.569 g. What is the true volume
Water was drained from a buret between the 0.12- and 15.78-mL marks. The apparent volume was 15.78 - 0.12 = 15.66 mL. Measured in air at 25 o C, the mass of water delivered was 15.569 g. What is the true volume
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter3: Matter-properties And Changes
Section: Chapter Questions
Problem 40A
Related questions
Question
Water was drained from a buret between the 0.12- and 15.78-mL marks. The
apparent volume was 15.78 - 0.12 = 15.66 mL. Measured in air at 25 o C, the mass of water delivered was 15.569 g. What is the true volume?
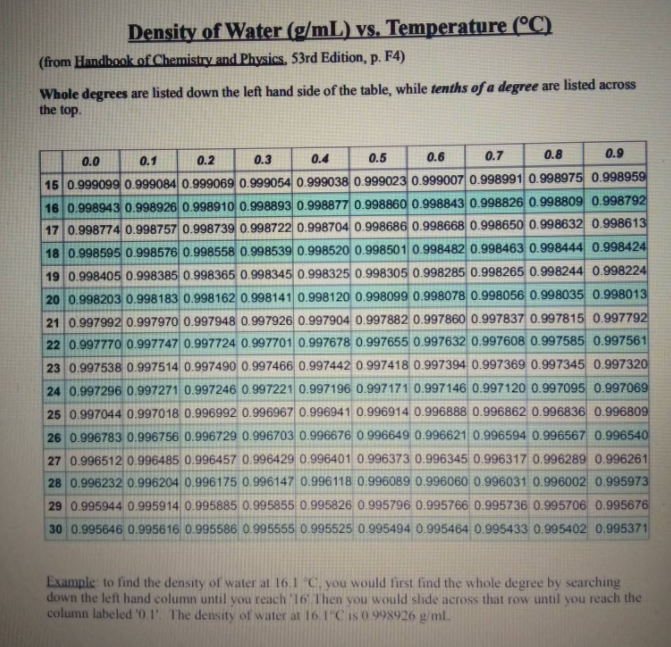
Transcribed Image Text:Density of Water (g/mL) vs. Temperature (°C)
(from Handbook of Chemistry and Physics. 53rd Edition, p. F4)
Whole degrees are listed down the left hand side of the table, while tenths of a degree are listed across
the top.
0.1
0.3
0.4
0.5
0.6
0.7
0.8
0.9
0.0
0.2
15 0.999099 0.999084 0.999069 0.999054 0.999038 0.999023 0.999007 0.9989910.998975 0.998959
16 0.998943 0.998926 0.998910 0.998893 0.998877 0.998860 0.998843 0.998826 0.998809 0.998792
17 0.998774 0.998757 0.998739 0.998722 0.998704 0.998686 0.998668 0.998650 0.998632 0.998613
18 0.998595 0.998576 0.998558 0.998539 0.998520 0.998501 0.998482 0.998463 0.998444 0.998424
19 0.998405 0.998385 0.998365 0.998345 0.998325 0.998305 0.998285 0.998265 0.998244 0.998224
20 0.998203 0.998183 0.998162 0.998141 0.998120 0.998099 0.998078 0.998056 0.998035 0.998013
21 0.997992 0.997970 0.997948 0.997926 0.997904 0.9978820.997860 0.997837 0.997815 0.997792
22 0.997770 0.997747 0.997724 0.997701 0.997678 0.997655 0.997632 0.997608 0.997585 0.997561
23 0.997538 0.997514 0.997490 0.997466 0.997442 0.997418 0.997394 0.997369 0.997345 0.997320
24 0.997296 0.997271 0.997246 0.997221 0.997196 0.997171 0.997146 0.997120 0.997095 0.997069
25 0.997044 0.997018 0.996992 0.996967 0.996941 0.996914 0.996888 0.996862 0.996836 0.996809
26 0.996783 0.996756 0.996729 0.996703 0.996676 0.996649 0.996621 0.996594 0.996567 0996540
27 0.996512 0.996485 0.996457 0.996429 0.996401 0.996373 0.996345 0.996317 0.996289 0.996261
28 0.996232 0.996204 0.996175 0.996147 0.996118 0.996089 0.996060 0.996031 0.996002 0.995973
29 0.995944 0.995914 0.995885 0.995855 0.995826 0.995796 0.995766 0.995736 0.995706 0.995676
30 0.995646 0.995616 0.995586 0.995555 0.995525 0.995494 0.995464 0.995433 0.995402 0.995371
Example to find the density of water at 16.1 C, you would first find the whole degree by searching
down the left hand column until you reach 16 Then you would slide across that row until you reach the
column labeled '0.1'. The density of water at 16.1"C is 0.998926 g/ml.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning