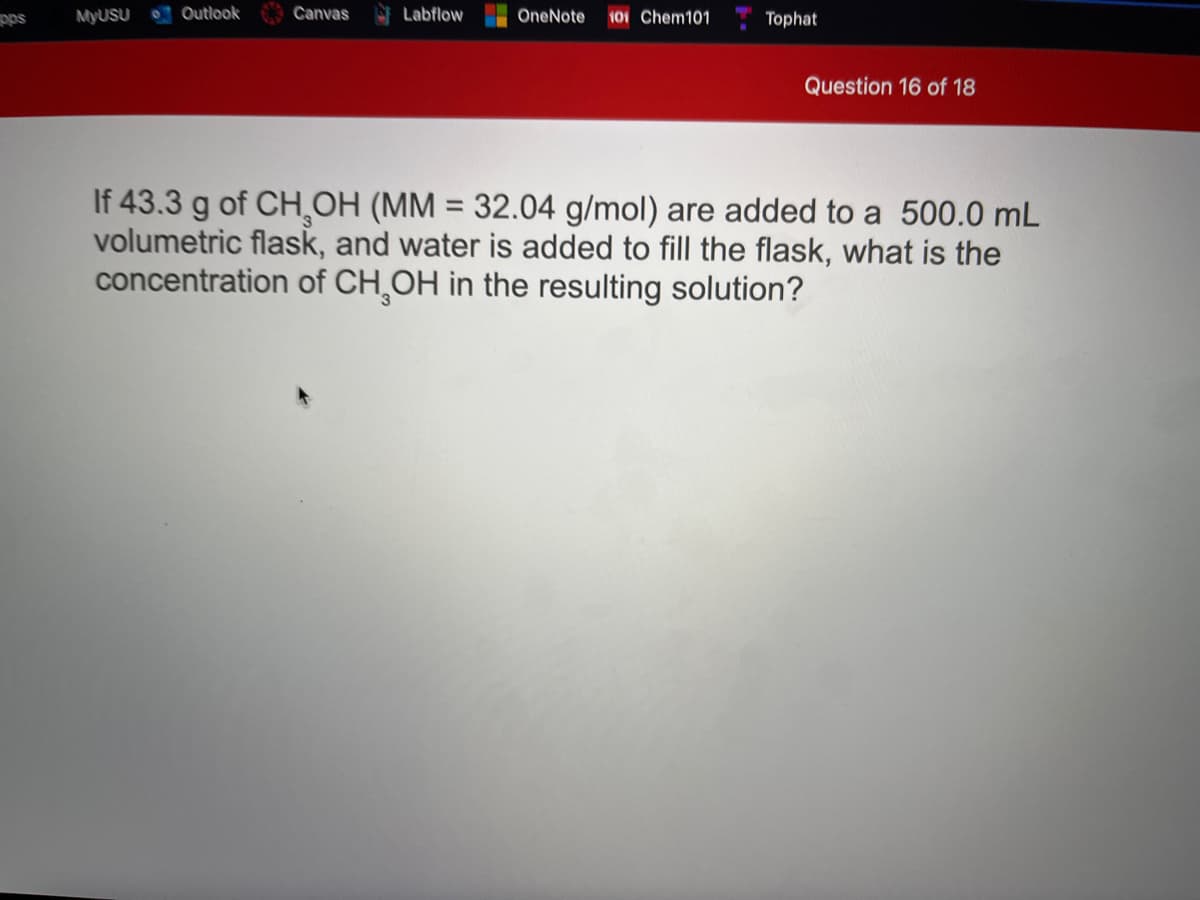
If 43.3 g of CHOH (MM = 32.04 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of CH¸OH in the resulting solution? %3D
If 43.3 g of CHOH (MM = 32.04 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of CH¸OH in the resulting solution? %3D
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter6: Solutions And Colloids
Section: Chapter Questions
Problem 6.53P: 6-53 Dioxin is considered to be poisonous in concentrations above 2 ppb. If a lake containing L has...
Related questions
Question
Transcribed Image Text:pps
MYUSU
Outlook
Canvas
Labflow
OneNote
101 Chem101
Tophat
Question 16 of 18
If 43.3 g of CHOH (MM = 32.04 g/mol) are added to a 500.0 mL
volumetric flask, and water is added to fill the flask, what is the
concentration of CH,OH in the resulting solution?
%3D
Expert Solution
Step 1
Definition:
Molarity is a concentration term for a solution. The molarity of a given solution is defined as the number of moles of solute present in one liter of solution. One molar is the molarity of a solution where one mole of solute is dissolved in a liter of solution.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning