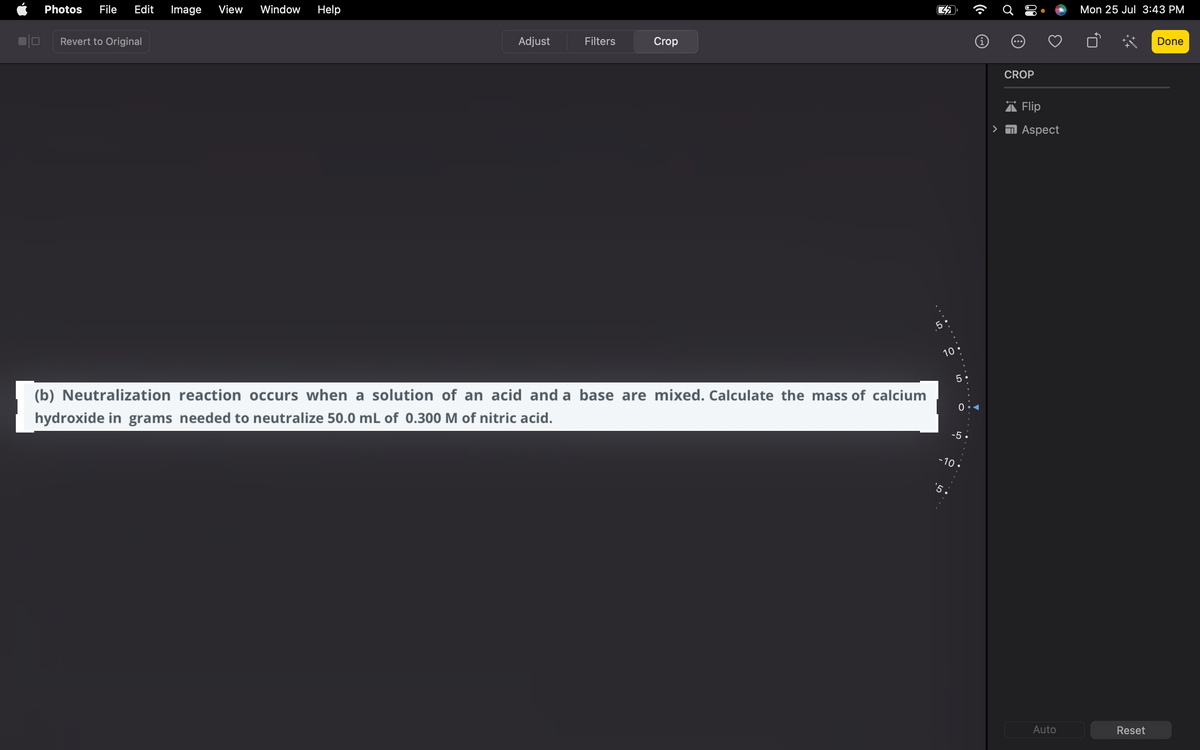
(b) Neutralization reaction occurs when a solution of an acid and a base are mixed. Calculate the mass of calcium hydroxide in grams needed to neutralize 50.0 mL of 0.300 M of nitric acid. 10. 5. 0 -5. -10.
(b) Neutralization reaction occurs when a solution of an acid and a base are mixed. Calculate the mass of calcium hydroxide in grams needed to neutralize 50.0 mL of 0.300 M of nitric acid. 10. 5. 0 -5. -10.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter18: Representative Metals, Metalloids, And Nonmetals
Section: Chapter Questions
Problem 115E: A mixture of xenon and ?uorine was heated. A sample of the white solid that formed reacted with...
Related questions
Question
2b
Transcribed Image Text:Photos File Edit Image View Window Help
Revert to Original
Adjust
Filters
Crop
(b) Neutralization reaction occurs when a solution of an acid and a base are mixed. Calculate the mass of calcium
hydroxide in grams needed to neutralize 50.0 mL of 0.300 M of nitric acid.
C
5
10.
5
5
0
-5
-10.
‚ . . .
CROP
Flip
Aspect
Auto
Mon 25 Jul 3:43 PM
Ö
Reset
Done
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning