If 45.3 mL of 0.116 M HC1 solution is needed to neutralize a solution of KOH, how many grams of KOH must be present in the solution? ? m = Submit Request Answer
If 45.3 mL of 0.116 M HC1 solution is needed to neutralize a solution of KOH, how many grams of KOH must be present in the solution? ? m = Submit Request Answer
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.26QAP
Related questions
Question
Transcribed Image Text:LJ Labflow - report: Report and Dat: x
N Mylab and Mastering
Course Home
b Answered: zinc metal is added to X
->
A openvellum.ecollege.com/course.html?courseld=16418591&OpenVellumHMAC=013628d899c889f48948ece53ef3ebff#10001
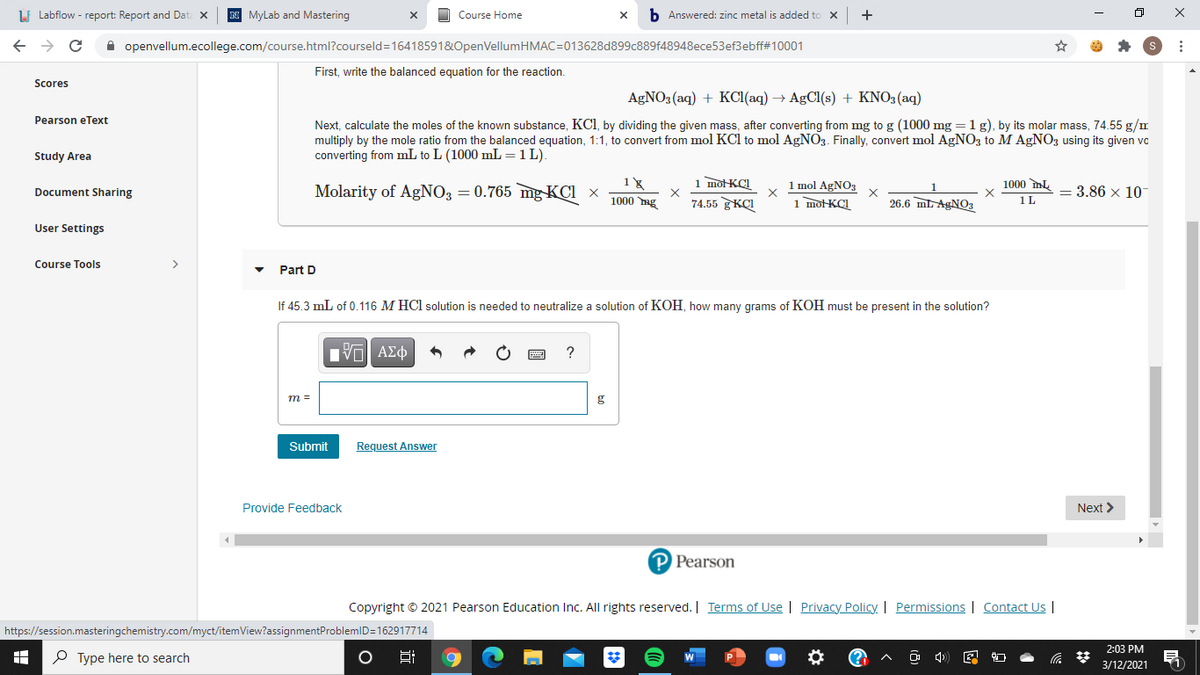
First, write the balanced equation for the reaction.
Scores
AGNO3 (aq) + KCI(aq) → AgCl(s) + KNO3(aq)
Pearson eText
Next, calculate the moles of the known substance, KCI, by dividing the given mass, after converting from mg to g (1000 mg =1g), by its molar mass, 74.55 g/m
multiply by the mole ratio from the balanced equation, 1:1, to convert from mol KCl to mol AGNO3. Finally, convert mol AgNO3 to M AgN03 using its given vo
converting from mL to L (1000 mL =1L).
Study Area
1 mot KÇI
1000 nL
Molarity of AgNO3 = 0.765 mg KCI ×
1 mol AgNO3
1 motKCI
Document Sharing
= 3.86 x 10
1000 ng
74.55 KCI
26.6 mL AGNO3
1L
User Settings
Course Tools
>
Part D
If 45.3 mL of 0.116 M HCl solution is needed to neutralize a solution of KOH, how many grams of KOH must be present in the solution?
?
т
Submit
Request Answer
Provide Feedback
Next >
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy. | Permissions | Contact Us |
https://session.masteringchemistry.com/myct/itemView?assignmentProblemID=162917714
2:03 PM
P Type here to search
3/12/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
