If 65.0 kg of ammonia and 80.0 kg of oxygen are fed to a batch reactor, determine the limiting reactant, the amount in moles by which the other reactant is in excess, and the extent of the reaction (molar) and the mass of NO produced (kg) if the reaction proceeds to completion. First find the number of moles that corresponds to 65.0 kg of ammonia (NH3). i kmol NH3 Now find the number of moles that corresponds to 80.0 kg of O2. i kmol O2
If 65.0 kg of ammonia and 80.0 kg of oxygen are fed to a batch reactor, determine the limiting reactant, the amount in moles by which the other reactant is in excess, and the extent of the reaction (molar) and the mass of NO produced (kg) if the reaction proceeds to completion. First find the number of moles that corresponds to 65.0 kg of ammonia (NH3). i kmol NH3 Now find the number of moles that corresponds to 80.0 kg of O2. i kmol O2
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 90E: The space shuttle Orbiter utilizes the oxidation of methylhydrazine by dinitrogen tetroxide for...
Related questions
Question
Use the extent of reaction e method and complete solution.
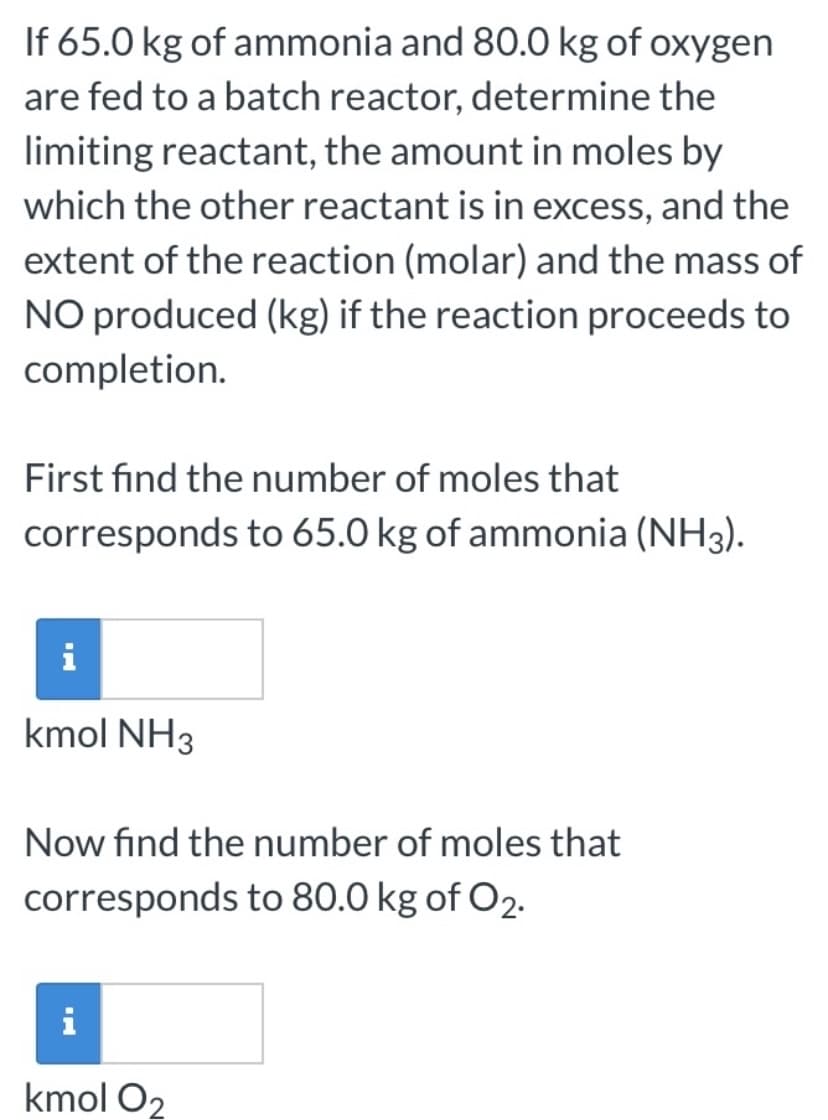
Transcribed Image Text:If 65.0 kg of ammonia and 80.0 kg of oxygen
are fed to a batch reactor, determine the
limiting reactant, the amount in moles by
which the other reactant is in excess, and the
extent of the reaction (molar) and the mass of
NO produced (kg) if the reaction proceeds to
completion.
First find the number of moles that
corresponds to 65.0 kg of ammonia (NH3).
i
kmol NH3
Now find the number of moles that
corresponds to 80.0 kg of O2.
i
kmol O2
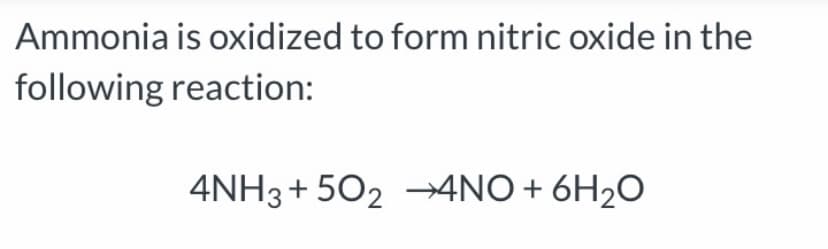
Transcribed Image Text:Ammonia is oxidized to form nitric oxide in the
following reaction:
4NH3+ 502 –→4NO+
6H2O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
