Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.101PAE: 12.101 An engineer working on a design to extract petroleum from a deep thermal reservoir wishes to...
Related questions
Question
The blue on the bottom go in the grey boxes. fill the grey boxes in.
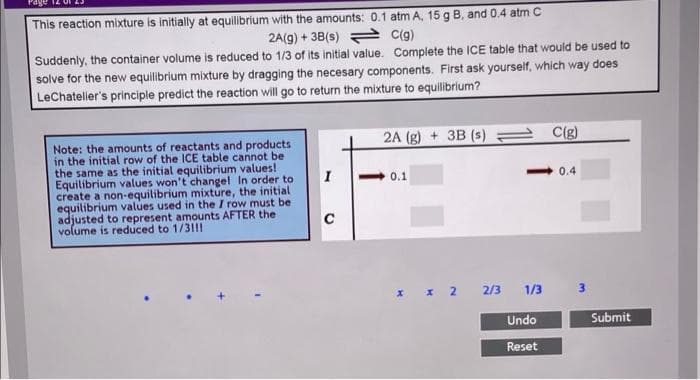
Transcribed Image Text:This reaction mixture is initially at equilibrium with the amounts: 0.1 atm A, 15 g B, and 0.4 atm C
2A(g) + 3B(s) = C(g)
Suddenly, the container volume is reduced to 1/3 of its initial value. Complete the ICE table that would be used to
solve for the new equilibrium mixture by dragging the necesary components. First ask yourself, which way does
LeChateler's principle predict the reaction will go to return the mixture to equilibrium?
2A (g) + 3B (s)
C(g)
Note: the amounts of reactants and products
in the initial row of the ICE table cannot be
the same as the initial equilibrium values!
Equilibrium values won't changel In order to
create a non-equilibrium mixture, the initial
equilibrium values used in the I row must be
adjusted to represent amounts AFTER the
volume is reduced to 1/31!!
I
0.1
0.4
C
2/3
1/3
Undo
Submit
Reset
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning