If a box is not needed, leave it blank. Use the table 'Standard Reduction Potentials' located in the "Tables', to predict if a reaction will occur when Cu metal is put into a 1M aqueous Ag* solution. Ifa reaction will occur, write a balanced net ionic equation for the reaction. If no reaction will occur, leave all boxes blank. |-[
If a box is not needed, leave it blank. Use the table 'Standard Reduction Potentials' located in the "Tables', to predict if a reaction will occur when Cu metal is put into a 1M aqueous Ag* solution. Ifa reaction will occur, write a balanced net ionic equation for the reaction. If no reaction will occur, leave all boxes blank. |-[
Related questions
Question
please answer the attached image. thank you!
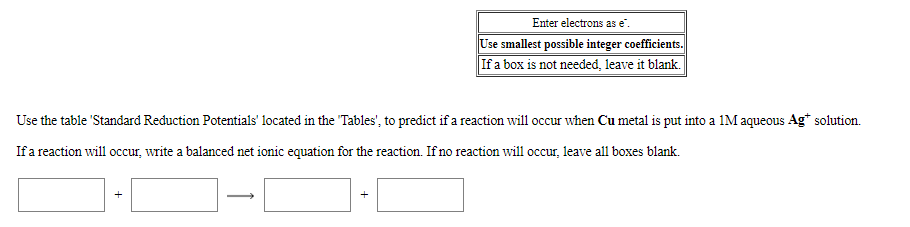
Transcribed Image Text:Enter electrons as e".
Use smallest possible integer coefficients.
If a box is not needed, leave it blank.
Use the table 'Standard Reduction Potentials' located in the "Tables", to predict if a reaction will occur when Cu metal is put into a 1M aqueous Ag* solution.
Ifa reaction will occur, write a balanced net ionic equation for the reaction. If no reaction will occur, leave all boxes blank.
+
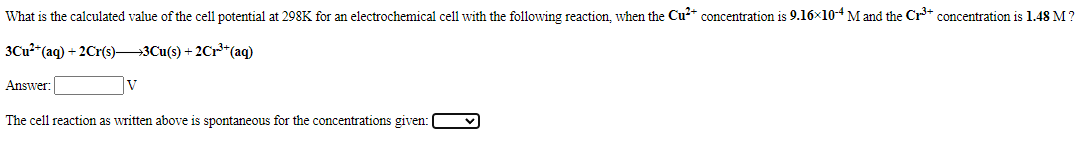
Transcribed Image Text:What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Cu* concentration is 9.16x10-4M and the Cr+ concentration is 1.48 M?
3Cu2 (aq) + 2Cr(s)3Cu(s) + 2Cr**(aq)
Answer:
V
The cell reaction as written above is spontaneous for the concentrations given:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps
