Use the table 'Standard Reduction Potentials' located in the "Tables', to predict if a reaction will occur when Cu metal is put into a 1M aqueous Ag* solution. If a reaction will occur, write a balanced net ionic equation for the reaction. If no reaction will occur, leave all boxes blank.
Use the table 'Standard Reduction Potentials' located in the "Tables', to predict if a reaction will occur when Cu metal is put into a 1M aqueous Ag* solution. If a reaction will occur, write a balanced net ionic equation for the reaction. If no reaction will occur, leave all boxes blank.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 160MP
Related questions
Question
Please answer. Thank you!
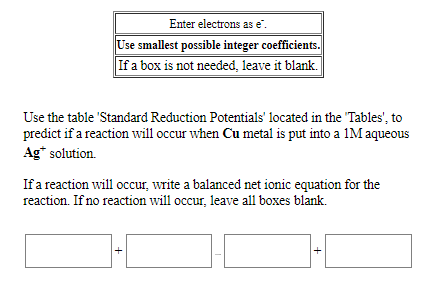
Transcribed Image Text:Enter electrons as e.
Use smallest possible integer coefficients.
If a box is not needed, leave it blank.
Use the table 'Standard Reduction Potentials' located in the "Tables', to
predict if a reaction will occur when Cu metal is put into a 1M aqueous
Ag* solution.
If a reaction will occur, write a balanced net ionic equation for the
reaction. If no reaction will occur, leave all boxes blank.
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning