If a student accurately made a measurement of 3.00, which of the following rulers must they have used (ignore units)? ں O 3 3 3 بلس 4 3.1 سلس 5 3.01 6 4 اسلىسسىلسا A student measure the volume of a liquid to be 41.311 mL. If the correct volume is 52.326 mL, what is their percent error? Type your answer rounded to the second decimal place without any units and without any + or - signs (i.e. NN.NN).
If a student accurately made a measurement of 3.00, which of the following rulers must they have used (ignore units)? ں O 3 3 3 بلس 4 3.1 سلس 5 3.01 6 4 اسلىسسىلسا A student measure the volume of a liquid to be 41.311 mL. If the correct volume is 52.326 mL, what is their percent error? Type your answer rounded to the second decimal place without any units and without any + or - signs (i.e. NN.NN).
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 101AE: The density of an irregularly shaped object was determined as follows. The mass of the object was...
Related questions
Question
Please send me the question in 20 minutes it's very urgent plz
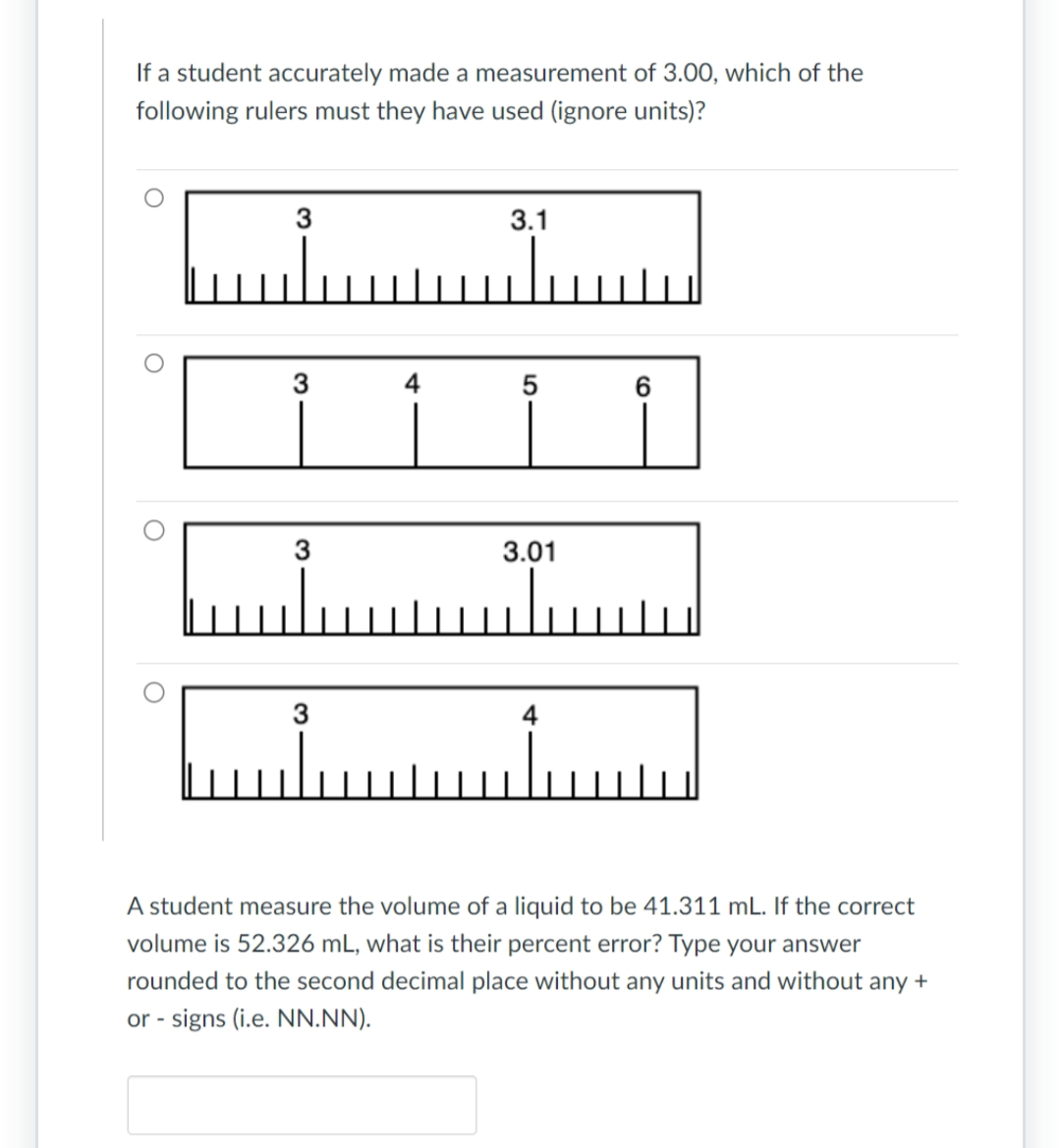
Transcribed Image Text:If a student accurately made a measurement of 3.00, which of the
following rulers must they have used (ignore units)?
O
3
3
3
3
4
3.1
Luu
5
3.01
4
6
A student measure the volume of a liquid to be 41.311 mL. If the correct
volume is 52.326 mL, what is their percent error? Type your answer
rounded to the second decimal place without any units and without any +
or - signs (i.e. NN.NN).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning