If the following reaction 2 C4H10 (g) + 13 O₂ (g) →8 CO₂ (g) + 10 H₂O(g) has AH = -115 kJ/mol and AS°= +248 J/K mol, how would the reaction be characterized with regards to its spontaneity? Periodic Table e Potentially Useful Information: AS n = (n*AS)products - (n*AS)reactants AG-AH-TAS AGxn- (n'AG)produts - (n'AG)reactants AG-AG + RT InQ K= e(Gorn/RT O spontaneous at all temperatures O spontaneous only at high temperature O spontaneous only at low temperature O nonspontaneous at all temperatures O unable to determine without more information Given the following table of thermodynamic data, AH, (kJ/mol) Substance H₂O2(g) H₂O2(0) for the following reaction being conducted at 298K H₂O2(1)→ H₂O2(g) 1. Calculate the AGºrxn -136.30 -187.80 answer yes or no AS (J/mol K) 232.60 110.00 kJ Report value to 4 sig figs 2. Is the reaction spontaneous under these standard state conditions?
If the following reaction 2 C4H10 (g) + 13 O₂ (g) →8 CO₂ (g) + 10 H₂O(g) has AH = -115 kJ/mol and AS°= +248 J/K mol, how would the reaction be characterized with regards to its spontaneity? Periodic Table e Potentially Useful Information: AS n = (n*AS)products - (n*AS)reactants AG-AH-TAS AGxn- (n'AG)produts - (n'AG)reactants AG-AG + RT InQ K= e(Gorn/RT O spontaneous at all temperatures O spontaneous only at high temperature O spontaneous only at low temperature O nonspontaneous at all temperatures O unable to determine without more information Given the following table of thermodynamic data, AH, (kJ/mol) Substance H₂O2(g) H₂O2(0) for the following reaction being conducted at 298K H₂O2(1)→ H₂O2(g) 1. Calculate the AGºrxn -136.30 -187.80 answer yes or no AS (J/mol K) 232.60 110.00 kJ Report value to 4 sig figs 2. Is the reaction spontaneous under these standard state conditions?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section: Chapter Questions
Problem 43PS: The ionization constant, Ka, for acetic acid is 1.8 105 at 25 C. What is the value of rG for this...
Related questions
Question
Do it all please otherwise don't touch it; leave it for other expert's

Transcribed Image Text:If the following reaction
2 C4H10 (8) + 13 O2 (g) → 8 CO₂ (g) + 10 H₂O (g)
has AH° -115 kJ/mol and AS°= +248 J/K mol, how would the reaction be
characterized with regards to its spontaneity?
Periodic Table e
Potentially Useful Information:
AS n = (n*AS)products - (n'AS reactants
AG=AH - TAS
AG rxn = (n AG)prodts - (n'AG)reactants
AGxn=AGrxn+ RT InQ
K= e(Gorn/RT
O spontaneous at all temperatures
O spontaneous only at high temperature
O spontaneous only at low temperature
O nonspontaneous at all temperatures
O unable to determine without more information
Given the following table of thermodynamic data,
ΔΗ,° (kJ/mol)
Substance
H₂O2(g)
H₂O2(1)
for the following reaction being conducted at 298K
H₂O2(1)→ H₂O2(g)
1. Calculate the AGºrn
-136.30
-187.80
answer yes or no
AS° (J/mol K)
232.60
110.00
kJ Report value to 4 sig figs
2. Is the reaction spontaneous under these standard state conditions?
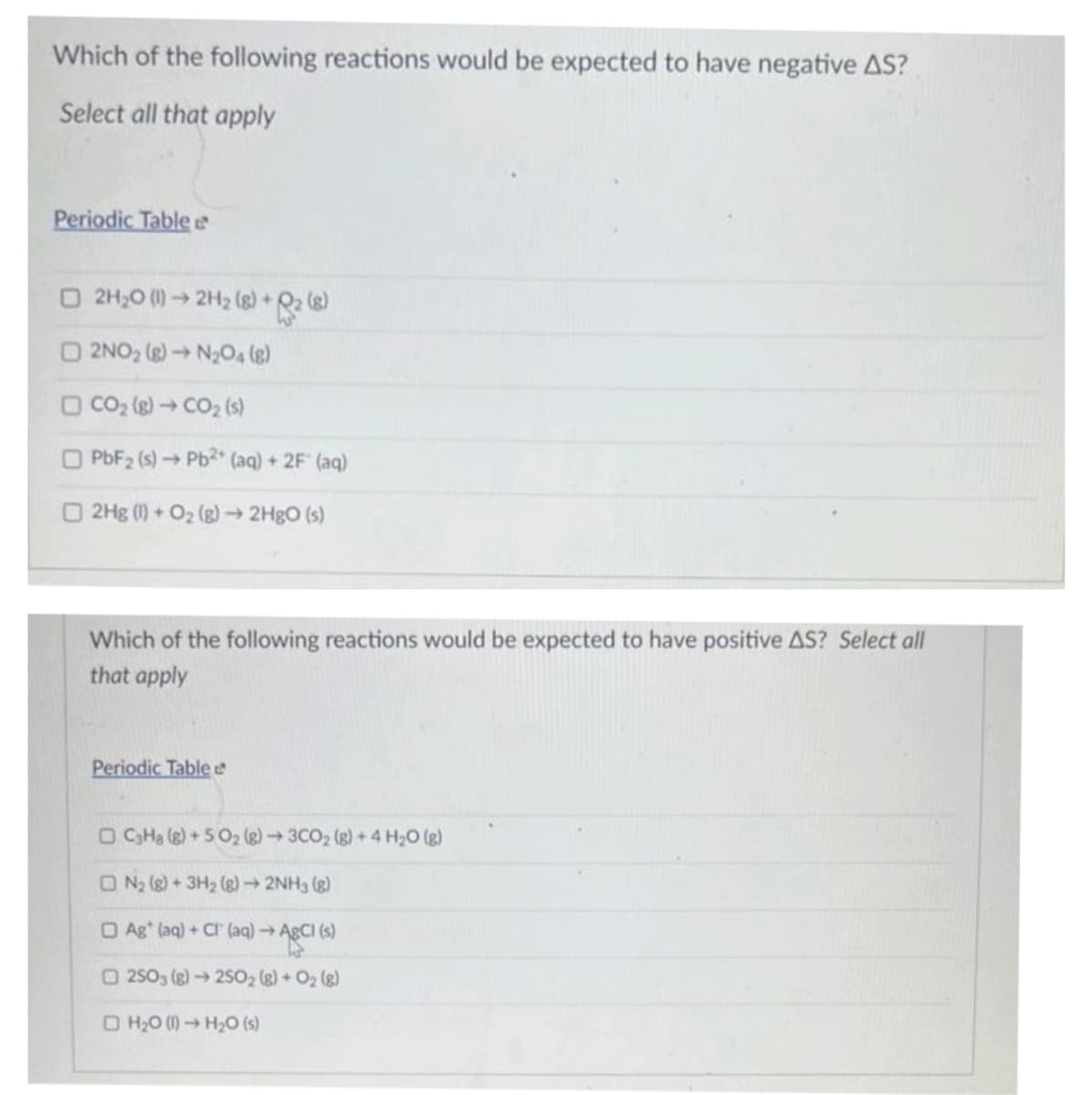
Transcribed Image Text:Which of the following reactions would be expected to have negative AS?
Select all that apply
Periodic Table
□ 2H₂0 (1)→ 2H₂ (8) +2 (8)
2NO₂ (8)→ N₂O4 (8)
□ CO₂ (g) → CO₂ (s)
O PbF₂ (s) → Pb2+ (aq) + 2F (aq)
2Hg (1) + O₂(g) → 2HgO (s)
Which of the following reactions would be expected to have positive AS? Select all
that apply
Periodic Table e
□ C3H8 (8) +50₂ (8)→ 3CO₂ (g) + 4H₂O(g)
ON₂ (8) + 3H₂ (8)→ 2NH3 (8)
Ag* (aq) + Cl(aq) → AgCl (s)
O2503 (g) → 25O2 (g) + O₂(g)
H₂0 (1)→ H₂O (s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning