If the pressure was decreased for the following reaction that was at equilibrium, how will the equilibrium position shift? N2(g) + Oz(g) <=> 2NO(g) Select one: O a. Right tion O b. No Shift Gasex O d. Cannot determine O e. Left Predict how the equilibrium position will shift if 100 grams of NaSCN is added to a solution containing the following at equilibrium? Fe*(aq) + SCN (aq) <=> FESCN? (aq) Select one: No shift stion O b. shift to the left O. shift to the right O d. Cannot determine
If the pressure was decreased for the following reaction that was at equilibrium, how will the equilibrium position shift? N2(g) + Oz(g) <=> 2NO(g) Select one: O a. Right tion O b. No Shift Gasex O d. Cannot determine O e. Left Predict how the equilibrium position will shift if 100 grams of NaSCN is added to a solution containing the following at equilibrium? Fe*(aq) + SCN (aq) <=> FESCN? (aq) Select one: No shift stion O b. shift to the left O. shift to the right O d. Cannot determine
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 9RQ: What is Le Chteliers principle? Consider the reaction 2NOCI(g)2NO(g)+Cl2(g) If this reaction is at...
Related questions
Question
Please I need help
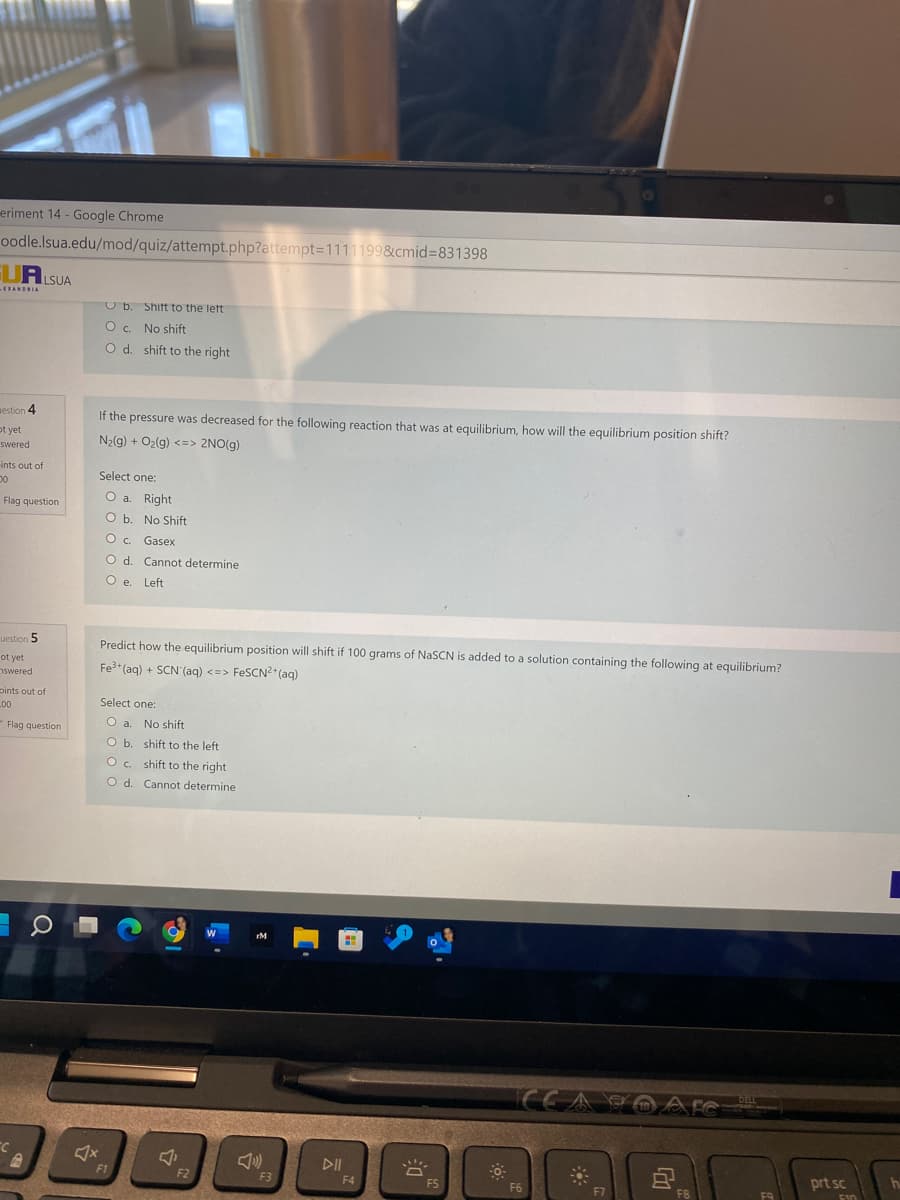
Transcribed Image Text:eriment 14 - Google Chrome
oodle.lsua.edu/mod/quiz/attempt.php?attempt=D1111199&cmid%=831398
UA LSUA
-EXANDRIA
U b. Shift to the let
O. No shift
O d. shift to the right
sestion 4
If the pressure was decreased for the following reaction that was at equilibrium, how will the equilibrium position shift?
ot yet
N2(g) + O2(g) <=> 2NO(g)
swered
ints out of
Select one:
O a. Right
Flag question
O b. No Shift
O. Gasex
O d. Cannot determine
O e. Left
uestion 5
Predict how the equilibrium position will shift if 100 grams of NaSCN is added to a solution containing the following at equilibrium?
ot yet
nswered
Fe (aq) + SCN'(aq) <=> FESCN²*(aq)
pints out of
L00
Select one:
- Flag question
O a. No shift
O b. shift to the left
O. shift to the right
O d. Cannot determine
rM
CCA TO
DELL
FC
DII
F1
prt sc
F2
F3
F4
F5
F6
F7
F8
F9
F10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning