If the uncatalyzed reaction occurs in a single elementary step, why is it a slow reaction? Check all that apply. • View Available Hint(s) All reactions that occur in one step are slow. The probability of an effective three-particle collision is low. The transition state is low in energy. The reaction requires the collision of three particles with the correct energy.
If the uncatalyzed reaction occurs in a single elementary step, why is it a slow reaction? Check all that apply. • View Available Hint(s) All reactions that occur in one step are slow. The probability of an effective three-particle collision is low. The transition state is low in energy. The reaction requires the collision of three particles with the correct energy.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 39QRT
Related questions
Question

Transcribed Image Text:If the uncatalyzed reaction occurs in a single elementary step, why is it a slow reaction?
Check all that apply.
• View Available Hint(s)
All reactions that occur in one step are slow.
The probability of an effective three-particle collision is low.
The transition state is low in energy.
The reaction requires the collision of three particles with the correct energy.
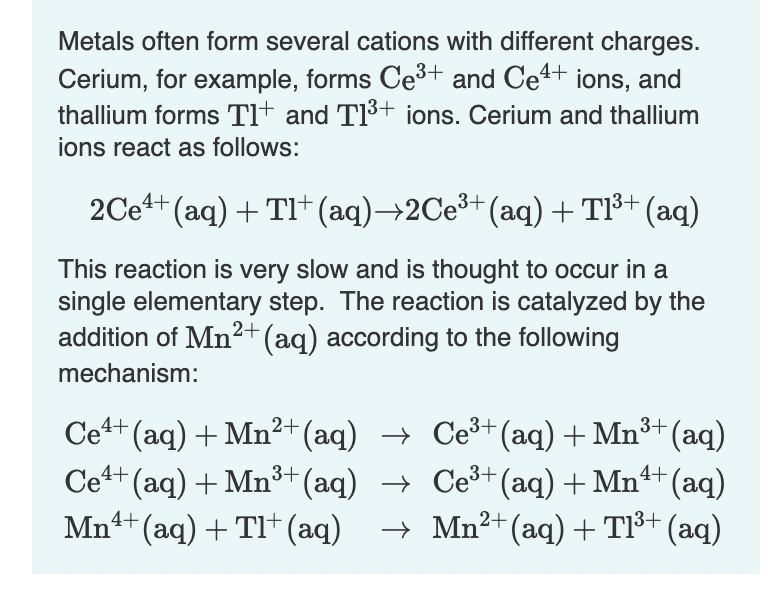
Transcribed Image Text:Metals often form several cations with different charges.
Cerium, for example, forms Ce3+ and Ce4+ ions, and
thallium forms Tlt and TI+ jons. Cerium and thallium
ions react as follows:
2Ce4+ (aq) + TI+ (aq)→2CE3+ (aq)+T³+ (aq)
This reaction is very slow and is thought to occur in a
single elementary step. The reaction is catalyzed by the
addition of Mn²+(aq) according to the following
mechanism:
Ce+ (aq) + Mn²+(aq) → Ce³+(aq) + Mn³+ (aq)
Ce+ (aq) + Mn³+(aq) → Ce³+(aq) + Mn+ (aq)
Mn1+ (aq) + TI+ (aq)
→ Mn2+ (aq) + Tl³+ (aq)
Expert Solution
Step 1
According to collision theory, the effective collisions are determined by activation energy and proper orientation.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax