your sketch d. On the graph, sketch the predicted results for the reactant if the volume of the reaction container is doubled. Explain. (You may assume this reaction is a non-zero order reaction.
your sketch d. On the graph, sketch the predicted results for the reactant if the volume of the reaction container is doubled. Explain. (You may assume this reaction is a non-zero order reaction.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 5ALQ: Consider the following statements: In general, the rate of a chemical reaction increases a bit at...
Related questions
Question
Only section d
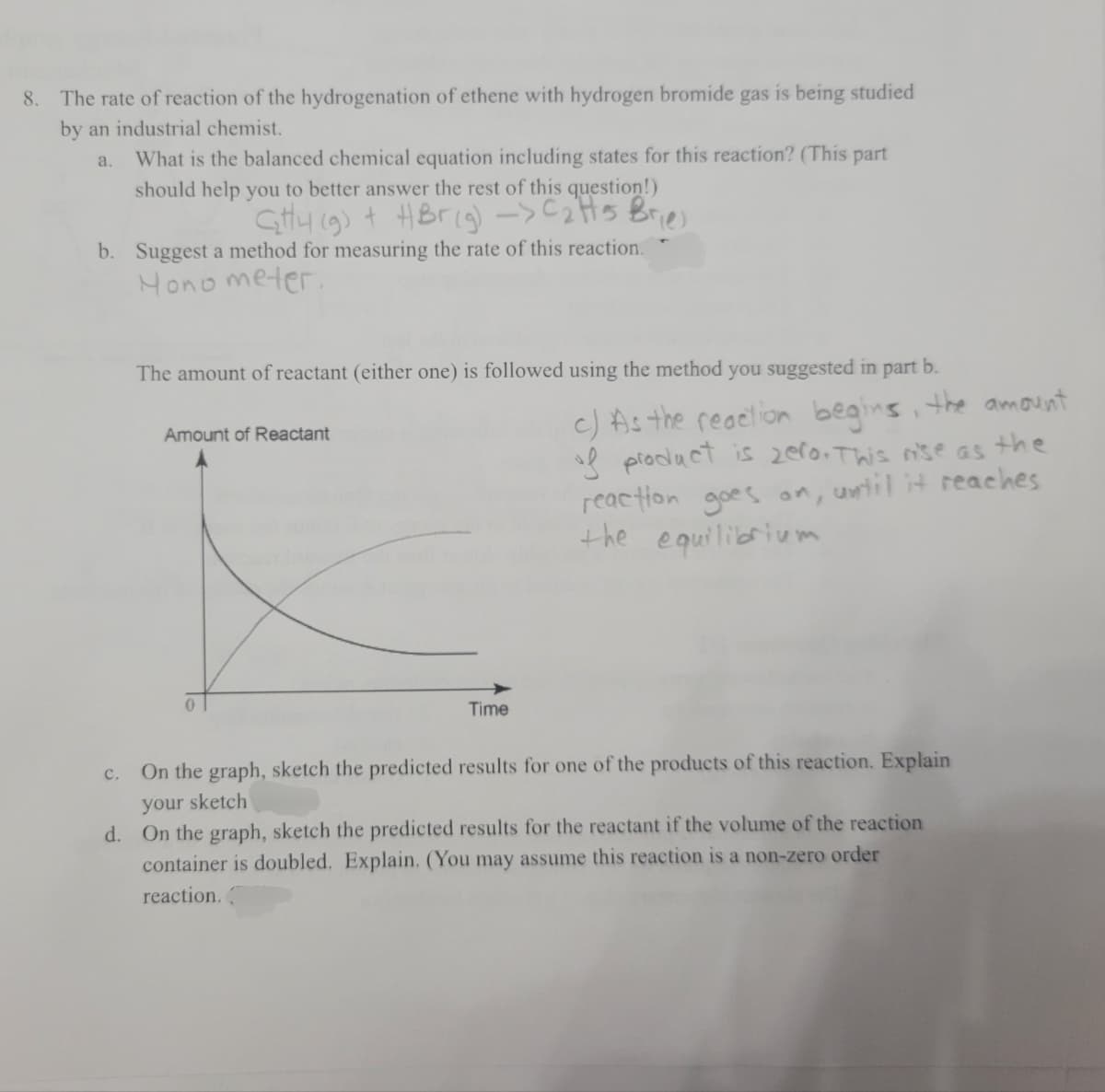
Transcribed Image Text:8. The rate of reaction of the hydrogenation of ethene with hydrogen bromide gas is being studied
by an industrial chemist.
What is the balanced chemical equation including states for this reaction? (This part
a.
should help you to better answer the rest of this question!)
Gtty 19) + HBrig)->C2HS Bries
b. Suggest a method for measuring the rate of this reaction.
Hono meter.
The amount of reactant (either one) is followed using the method you suggested in part b.
c) As the reaelion begins, the amount
sl piodact is zer0. This nse as the
reaction goes on, until it reaches
the equilibrium
Amount of Reactant
Time
c. On the graph, sketch the predicted results for one of the products of this reaction. Explain
your sketch
d. On the graph, sketch the predicted results for the reactant if the volume of the reaction
container is doubled. Explain. (You may assume this reaction is a non-zero order
reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning