If we need 80mL of 0.3M NaCI, it is harder to calculate. For any dilution we can use the formula C,V1 = C2V2. Using this formula, how many mL of 5M NaCl do you need to make the specificed solution?
If we need 80mL of 0.3M NaCI, it is harder to calculate. For any dilution we can use the formula C,V1 = C2V2. Using this formula, how many mL of 5M NaCl do you need to make the specificed solution?
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
need as soon as possibledon't copy from other sources I will downvote
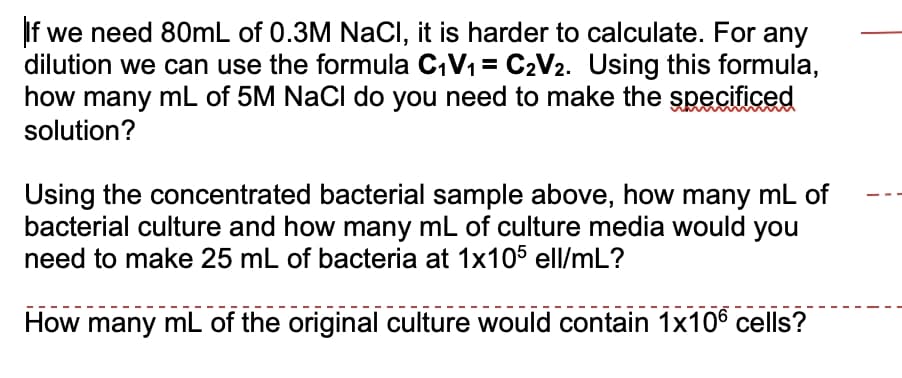
Transcribed Image Text:If we need 80mL of 0.3M NaCI, it is harder to calculate. For any
dilution we can use the formula C,V1 = C2V2. Using this formula,
how many mL of 5M NaCl do you need to make the specificed
solution?
Using the concentrated bacterial sample above, how many mL of
bacterial culture and how many mL of culture media would you
need to make 25 mL of bacteria at 1x105 ell/mL?
How many mL of the original culture would contain 1x106 cells?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

