Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 4RQ: Molarity is a conversion factor relating moles of solute in solution to the volume of the solution....
Related questions
Question
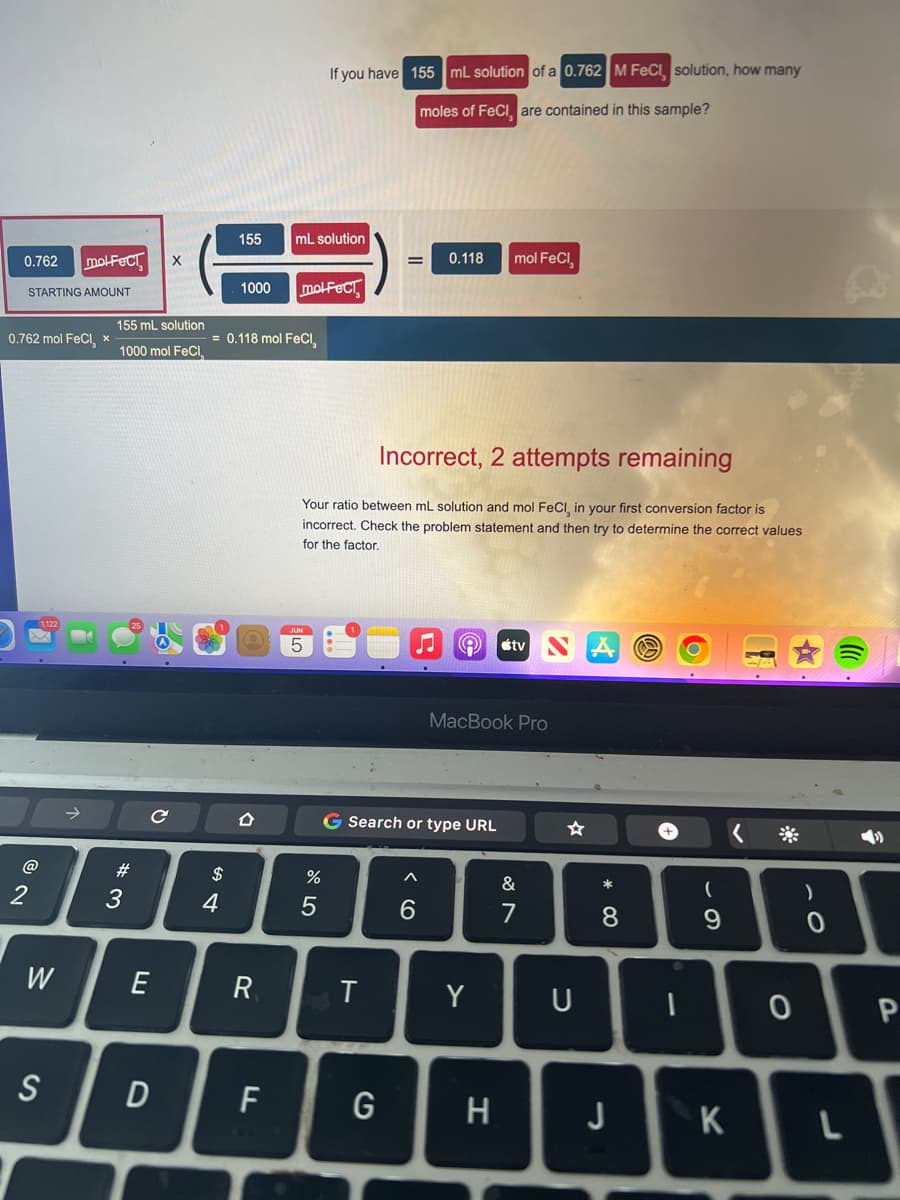
Transcribed Image Text:0.762 mol-FECT X
STARTING AMOUNT
0.762 mol FeCl, *
2
W
S
155 mL solution
1000 mol FeCl
#3
с
E
D
155
1000
= 0.118 mol FeCl,
4
R
If you have 155 mL solution of a 0.762 M FeCl, solution, how many
moles of FeCl, are contained in this sample?
mL solution
0.118 mol FeCl₂
molFECT
Incorrect, 2 attempts remaining
Your ratio between mL solution and mol FeCl, in your first conversion factor is
incorrect. Check the problem statement and then try to determine the correct values
for the factor.
5
9
tv N
S
O
MacBook Pro
F
G Search or type URL
%
^
5
T
G
6
Y
H
&
7
U
*
8
J
I
9
K
0
)
0
((((
L
3
P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div