(ii) Irradiation of diene A at 254 nm affords a photostationary mixture of A with triene B. Provide a mechanism for the formation of B and define the stereochemistry of the alkenes, explaining this stereochemical outcome by reference to the interacting frontier molecular orbitals. hv
(ii) Irradiation of diene A at 254 nm affords a photostationary mixture of A with triene B. Provide a mechanism for the formation of B and define the stereochemistry of the alkenes, explaining this stereochemical outcome by reference to the interacting frontier molecular orbitals. hv
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 73AP: Use your knowledge of directing effects, along with the following data, to deduce the directions of...
Related questions
Question
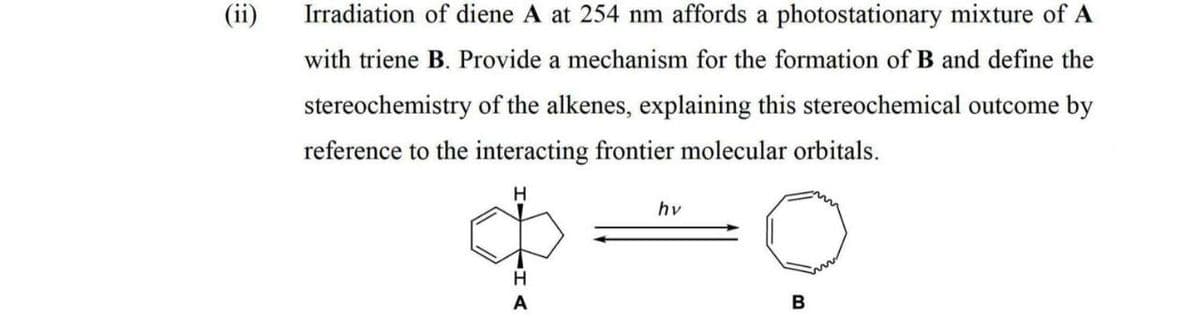
Transcribed Image Text:Irradiation of diene A at 254 nm affords a photostationary mixture of A
with triene B. Provide a mechanism for the formation of B and define the
stereochemistry of the alkenes, explaining this stereochemical outcome by
reference to the interacting frontier molecular orbitals.
H.
hv
H
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

