a) 67% What splitting pattern in the H NMR spectrum would you expect for all the hydrogen atoms in the compounds shown below? 2.2 C) 10/09 F10 H₂C F11 H₂C H IO CH CH3 CH2CH3 CH₂ F12 H₂ (9) Use a table of chemical shift values to describe fully how 1¹°C NMR spectroscopy can be used to distinguish between ethanol and methoxymethane. CH3 OH Insert D PrntScr
a) 67% What splitting pattern in the H NMR spectrum would you expect for all the hydrogen atoms in the compounds shown below? 2.2 C) 10/09 F10 H₂C F11 H₂C H IO CH CH3 CH2CH3 CH₂ F12 H₂ (9) Use a table of chemical shift values to describe fully how 1¹°C NMR spectroscopy can be used to distinguish between ethanol and methoxymethane. CH3 OH Insert D PrntScr
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
It looks like you may have submitted a graded question that, per our Honor Code, experts cannot answer. We've credited a question to your account.
Your Question:
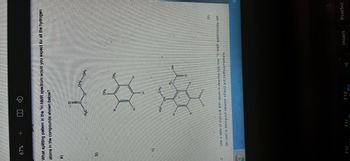
Transcribed Image Text:a)
67%
What splitting pattern in the H NMR spectrum would you expect for all the hydrogen
atoms in the compounds shown below?
2.2
C)
10/09
F10
H₂C
F11
H₂C
H
IO
CH
CH3
CH2CH3
CH₂
F12
H₂
(9)
Use a table of chemical shift values to describe fully how 1¹°C NMR spectroscopy can
be used to distinguish between ethanol and methoxymethane.
CH3
OH
Insert
D
PrntScr
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY
