II. While there are many different types of inhibitors, most have a very similar structure so they can compete with the normal substrate (in this case dopamine). Of the molecules below, which compound do you think has the most promise as an inhibitor? HO a.a.#1 N- H a.a.#2 H₂PO3 -PO₂H₂ OH Alendronate a.a.#6 NH₂ Carbidopa Using the same, amino acid orientation as you arrived at for Part I, show which inhibitor is most promising a.a.#5 Raloxifene a.a.#3 a.a.#4
II. While there are many different types of inhibitors, most have a very similar structure so they can compete with the normal substrate (in this case dopamine). Of the molecules below, which compound do you think has the most promise as an inhibitor? HO a.a.#1 N- H a.a.#2 H₂PO3 -PO₂H₂ OH Alendronate a.a.#6 NH₂ Carbidopa Using the same, amino acid orientation as you arrived at for Part I, show which inhibitor is most promising a.a.#5 Raloxifene a.a.#3 a.a.#4
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter22: Gluconeogenesis, Glycogen Metabolism, And The Pentose Phosphate Pathway
Section: Chapter Questions
Problem 23P: Using the ActiveModel for aldose reductase, describe the structure of the TIM barrel motif and the...
Related questions
Question
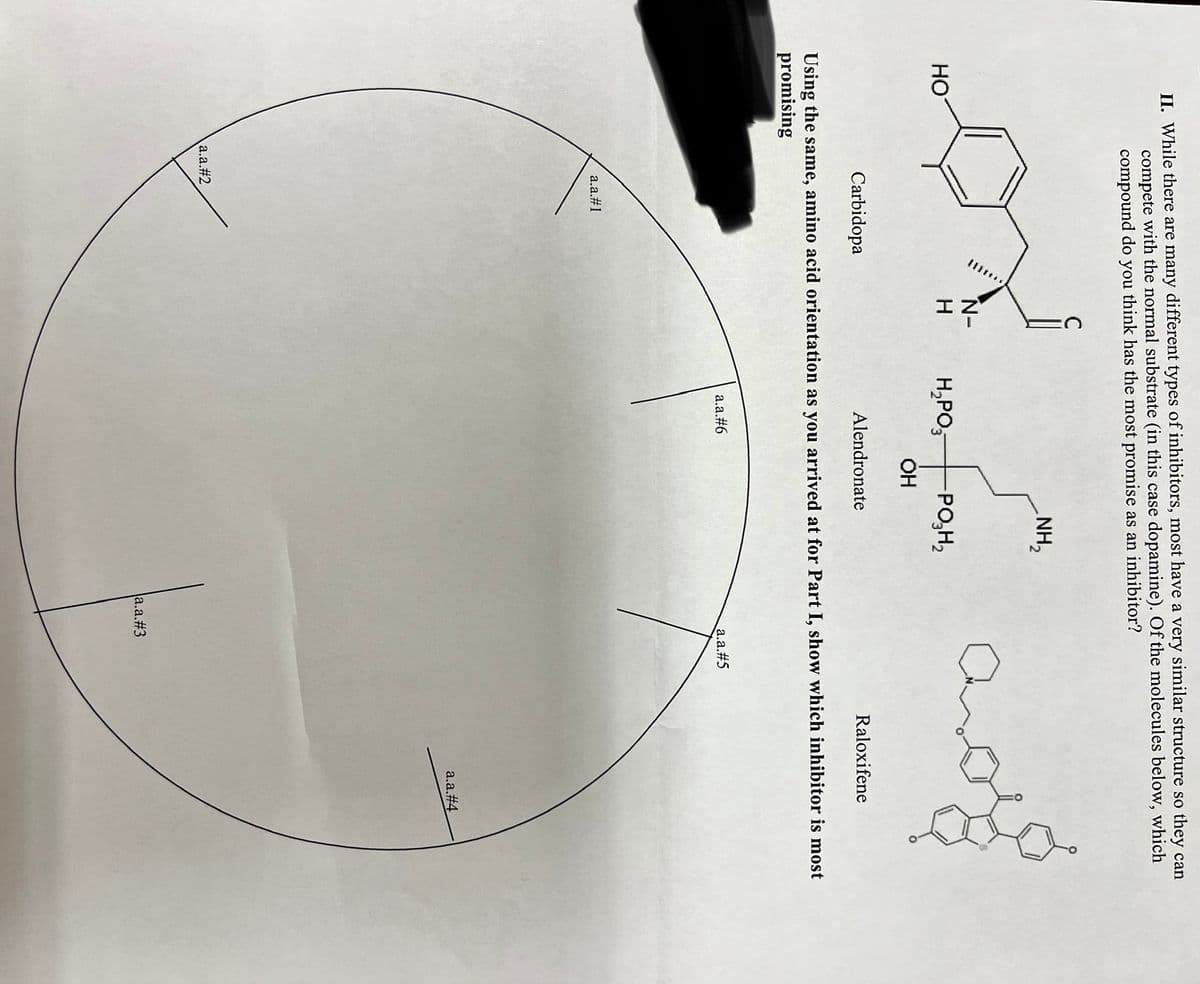
Transcribed Image Text:II. While there are many different types of inhibitors, most have a very similar structure so they can
compete with the normal substrate (in this case dopamine). Of the molecules below, which
compound do you think has the most promise as an inhibitor?
HO
a.a.#1
C
a.a.#2
N-
H
H₂PO
OH
Alendronate
a.a.#6
NH₂
-PO₂H₂
Carbidopa
Using the same, amino acid orientation as you arrived at for Part I, show which inhibitor is most
promising
Car
a.a.#5
a.a.#3
Raloxifene
a.a.#4
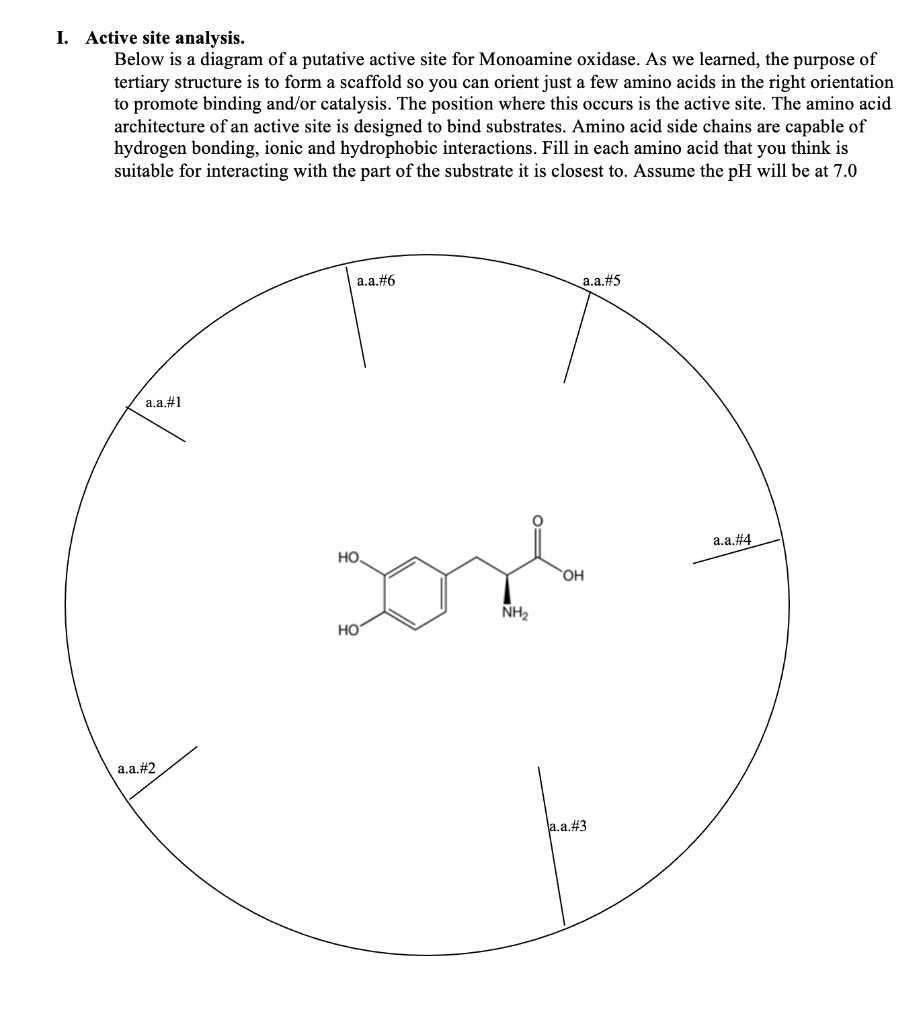
Transcribed Image Text:I. Active site analysis.
Below is a diagram of a putative active site for Monoamine oxidase. As we learned, the purpose of
tertiary structure is to form a scaffold so you can orient just a few amino acids in the right orientation
to promote binding and/or catalysis. The position where this occurs is the active site. The amino acid
architecture of an active site is designed to bind substrates. Amino acid side chains are capable of
hydrogen bonding, ionic and hydrophobic interactions. Fill in each amino acid that you think is
suitable for interacting with the part of the substrate it is closest to. Assume the pH will be at 7.0
a.a.#1
a.a.#2
a.a.#6
HO
Lond
NH₂
НО
a.a.#5
OH
a.a.#3
a.a.#4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning