In a nuclear fission reaction a heavy nucleus divides to form smaller nuclei and one or more neutrons. Many nuclei can undergo fission, but the fission reactions of uranium-235 and plutonium-239 are the principal ones that generate energy in nuclear power plants. This problem deals with balancing the fission reaction of the uranium-235 isotope as it undergoes bombardment from a neutron. Part A When a 235 U nucleus is bombarded by neutrons (n) it undergoes a fission reaction, resulting in the formation of two new nuclei and neutrons. The following equation is an example of one such fission process: 2U+n+Ba+ Kr +3n Enter the isotope symbol for the barium (Ba) nucleus in this reaction. Express your answer as an isotope. ► View Available Hint(s) _ | ΑΣΦ A chemical reaction does not occur for this question. Submit Part B ? In another process in which 235 U undergoes neutron bombardment, the following reaction occurs: 25U+nSr +54 Xe + 3n Enter the isotope symbol for the strontium (Sr) nucleus in this reaction. Express your answer as an isotope. ►View Available Hint(s) °= | ΑΣΦ ? A chemical reaction does not occur for this question.
In a nuclear fission reaction a heavy nucleus divides to form smaller nuclei and one or more neutrons. Many nuclei can undergo fission, but the fission reactions of uranium-235 and plutonium-239 are the principal ones that generate energy in nuclear power plants. This problem deals with balancing the fission reaction of the uranium-235 isotope as it undergoes bombardment from a neutron. Part A When a 235 U nucleus is bombarded by neutrons (n) it undergoes a fission reaction, resulting in the formation of two new nuclei and neutrons. The following equation is an example of one such fission process: 2U+n+Ba+ Kr +3n Enter the isotope symbol for the barium (Ba) nucleus in this reaction. Express your answer as an isotope. ► View Available Hint(s) _ | ΑΣΦ A chemical reaction does not occur for this question. Submit Part B ? In another process in which 235 U undergoes neutron bombardment, the following reaction occurs: 25U+nSr +54 Xe + 3n Enter the isotope symbol for the strontium (Sr) nucleus in this reaction. Express your answer as an isotope. ►View Available Hint(s) °= | ΑΣΦ ? A chemical reaction does not occur for this question.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter25: Nuclear Chemistry
Section: Chapter Questions
Problem 51GQ
Related questions
Question
100%
22) please see picture
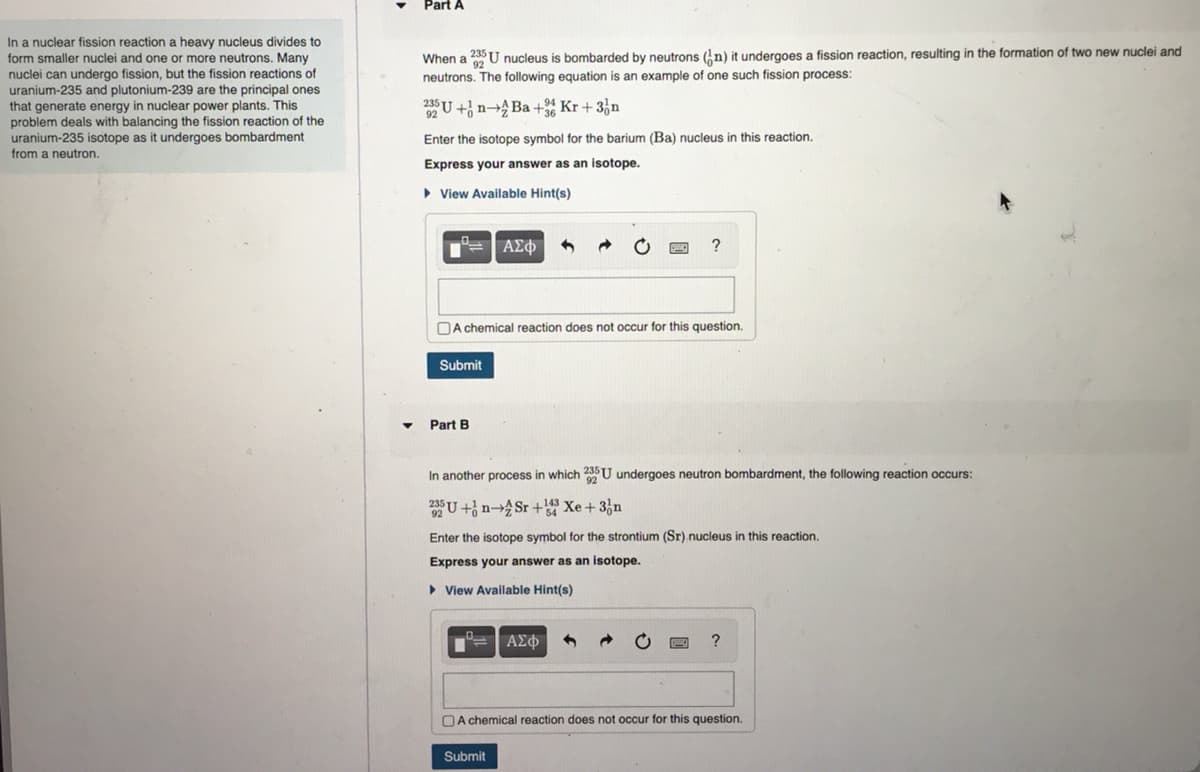
Transcribed Image Text:In a nuclear fission reaction a heavy nucleus divides to
form smaller nuclei and one or more neutrons. Many
nuclei can undergo fission, but the fission reactions of
uranium-235 and plutonium-239 are the principal ones
that generate energy in nuclear power plants. This
problem deals with balancing the fission reaction of the
uranium-235 isotope as it undergoes bombardment
from a neutron.
Part A
When a 23 U nucleus is bombarded by neutrons (n) it undergoes a fission reaction, resulting in the formation of two new nuclei and
neutrons. The following equation is an example of one such fission process:
25 U+ n-Ba +36 Kr + 3n
Enter the isotope symbol for the barium (Ba) nucleus in this reaction.
Express your answer as an isotope.
▸ View Available Hint(s)
°= | ΑΣΦ
A chemical reaction does not occur for this question.
Submit
Part B
?
In another process in which 225U undergoes neutron bombardment, the following reaction occurs:
3U+ồn—£Sr+33 Xe+3ần
Enter the isotope symbol for the strontium (Sr) nucleus in this reaction.
Express your answer as an isotope.
►View Available Hint(s)
— | ΑΣΦ
Submit
?
A chemical reaction does not occur for this question.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning