In an adiabatic process, a gas is compressed to ½ its volume and 1800 joules of work is done on the gas. How much heat enters or leaves the gas, and how much does the internal energy of the gas change? +1800 and 0 Joules -1800 and 0 Joules O and -1800 Joules O and +1800 Joules
In an adiabatic process, a gas is compressed to ½ its volume and 1800 joules of work is done on the gas. How much heat enters or leaves the gas, and how much does the internal energy of the gas change? +1800 and 0 Joules -1800 and 0 Joules O and -1800 Joules O and +1800 Joules
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 58P: Consider the processes shown below. In the processes AB and BC, 3600 J and 2400 J of heat are added...
Related questions
Question
Need help on question 7
Transcribed Image Text:W Thermodynar x
b Temperature
O Momentum Cx
I Emilio Martin
H Aeries | San E x
O Heat and The x
b During the la X
5/604f9765d9bb50dc16a01ced
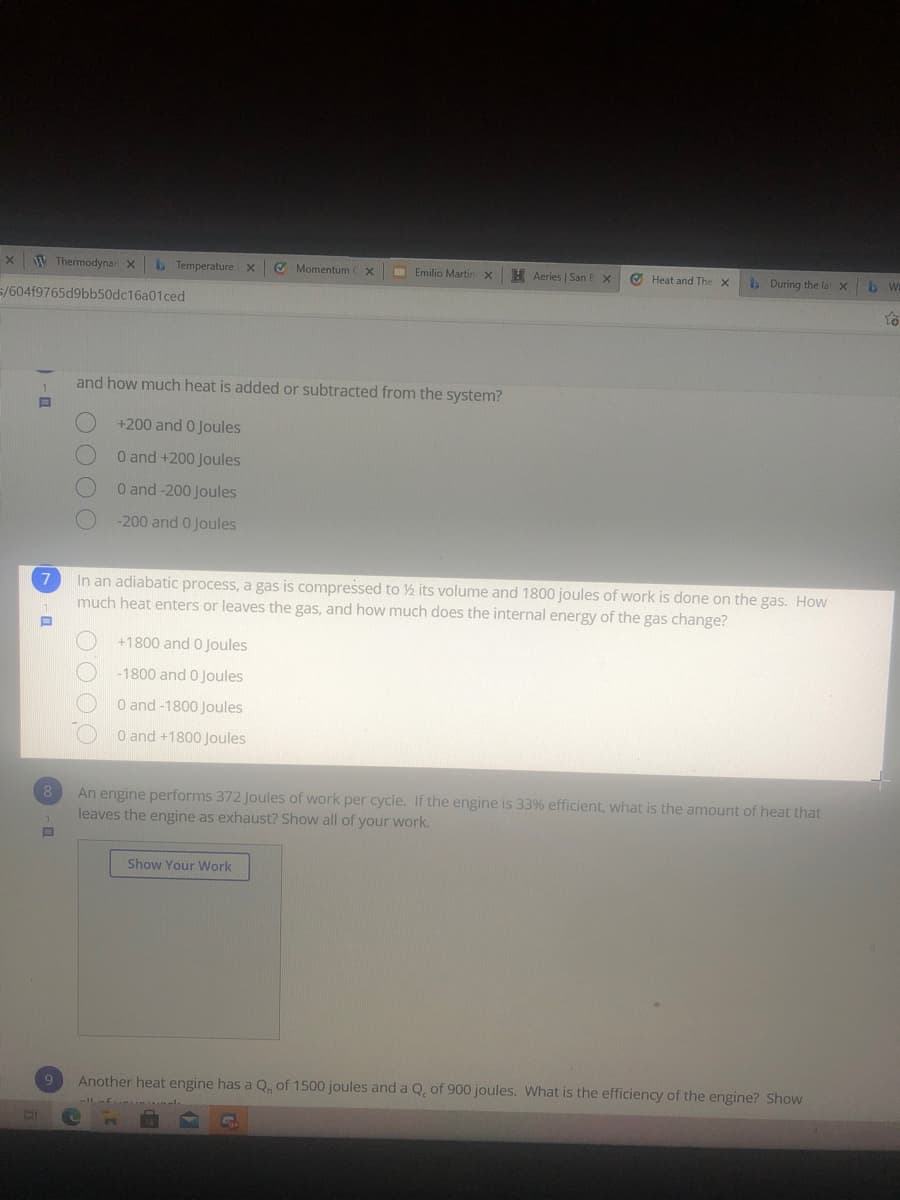
and how much heat is added or subtracted from the system?
+200 and 0 Joules
0 and +200 Joules
O and -200 Joules
-200 and 0 Joules
In an adiabatic process, a gas is compressed to ½ its volume and 1800 joules of work is done on the gas. How
much heat enters or leaves the gas, and how much does the internal energy of the gas change?
+1800 and 0 Joules
-1800 and 0 Joules
O and-1800 Joules
O and +1800 Joules
An engine performs 372 Joules of work per cycle. If the engine is 33% efficient, what is the amount of heat that
leaves the engine as exhaust? Show all of your work.
8
Show Your Work
9
Another heat engine has a Q. of 1500 joules and a Q, of 900 joules. What is the efficiency of the engine? Show
O OOO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning