In an experiment, 190 g of aluminum (with a specific heat of 900 J/kg•K) at 53.0°C is mixed with 81.0 g of water (with a specific heat of 4186 J/kg-K) at 13.0°C, with the mixture thermally isolated. (a) What is the equilibrium temperature? What are the entropy changes of (b) the aluminum, (c) the water, and (d) the aluminum-water system? (a) Number Units (b) Number -14.5521 Units J/K (c) Number 15.4953 Units J/K (d) Number i Units J/K
In an experiment, 190 g of aluminum (with a specific heat of 900 J/kg•K) at 53.0°C is mixed with 81.0 g of water (with a specific heat of 4186 J/kg-K) at 13.0°C, with the mixture thermally isolated. (a) What is the equilibrium temperature? What are the entropy changes of (b) the aluminum, (c) the water, and (d) the aluminum-water system? (a) Number Units (b) Number -14.5521 Units J/K (c) Number 15.4953 Units J/K (d) Number i Units J/K
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter22: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 80PQ
Related questions
Question
Please help me
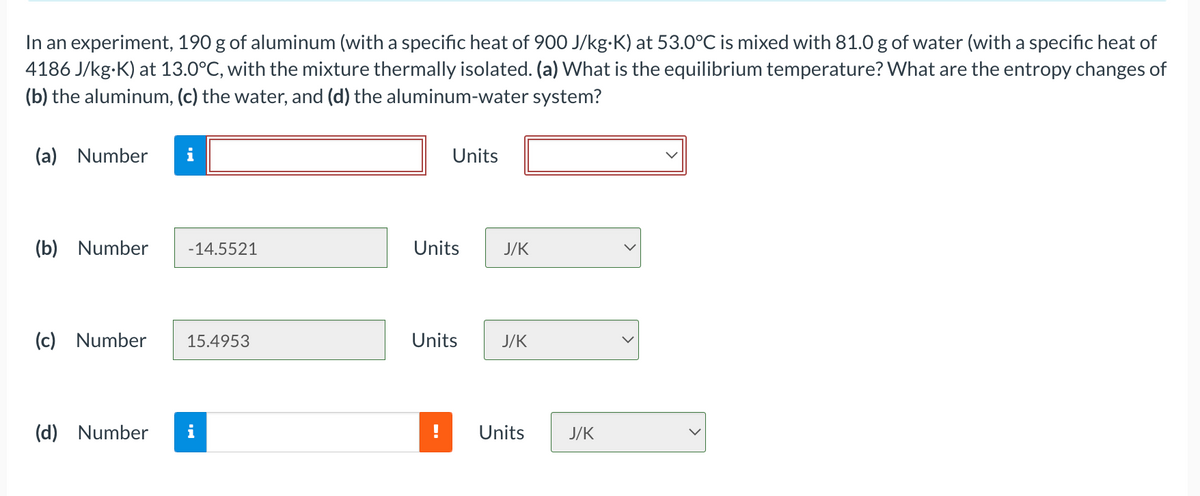
Transcribed Image Text:In an experiment, 190 g of aluminum (with a specific heat of 900 J/kg-K) at 53.0°C is mixed with 81.0 g of water (with a specific heat of
4186 J/kg-K) at 13.0°C, with the mixture thermally isolated. (a) What is the equilibrium temperature? What are the entropy changes of
(b) the aluminum, (c) the water, and (d) the aluminum-water system?
(a) Number
i
Units
(b) Number
-14.5521
Units
J/K
(c) Number
15.4953
Units
J/K
(d) Number
Units
J/K
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning