In an ionic equation, which types of species would always be shown as separate ions (ionized or dissociated) in the aqueous phase. Select all that apply. O strong bases strong acids ionic compounds molecular compounds weak bases weak acids
In an ionic equation, which types of species would always be shown as separate ions (ionized or dissociated) in the aqueous phase. Select all that apply. O strong bases strong acids ionic compounds molecular compounds weak bases weak acids
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 79AP
Related questions
Question
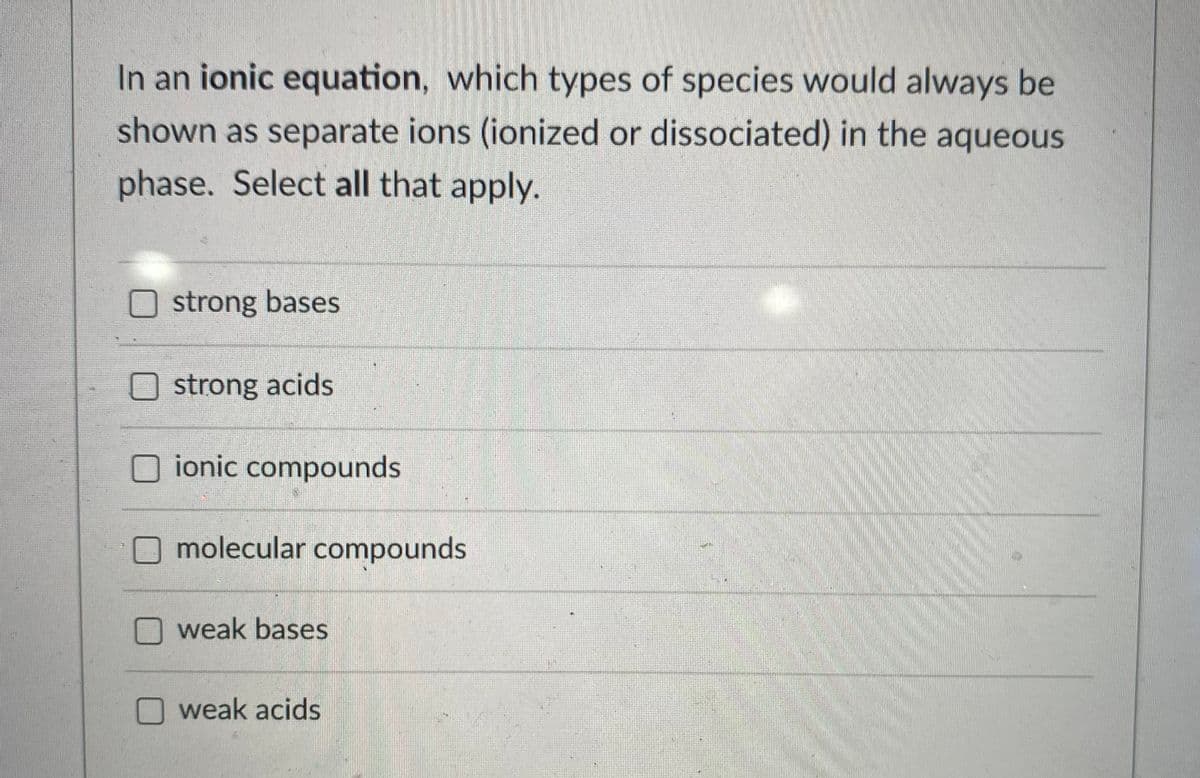
Transcribed Image Text:In an ionic equation, which types of species would always be
shown as separate ions (ionized or dissociated) in the aqueous
phase. Select all that apply.
strong bases
Ostrong acids
ionic compounds
O molecular compounds
weak bases
weak acids
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning