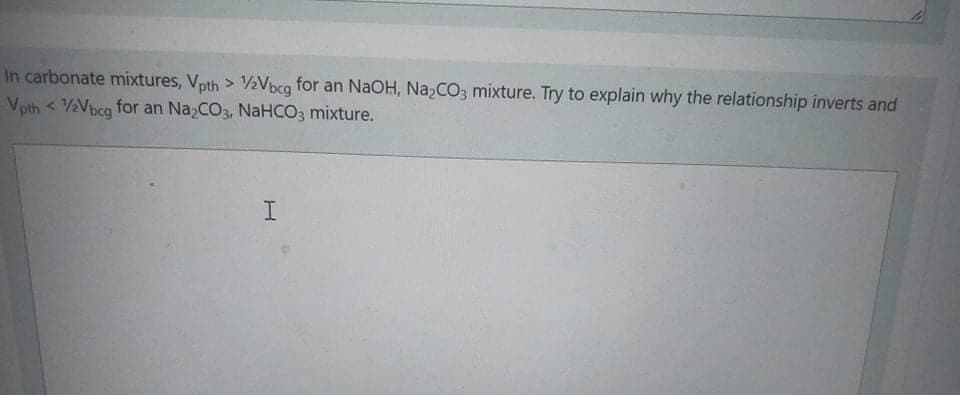
In carbonate mixtures, Vpth > ½Vbcg for an NaOH, N22CO3 mixture. Try to explain why the relationship inverts and Vpth < V2Vbcg for an Na;CO3, NaHCO3 mixture.
Q: In the labo E obtains tlhe following data for an aqueous solution of KCI (molar mass = 74.6 g/mol)…
A: Molality of a solution = moles of solute/mass of solvent in kg % w/v concentration = [mass of…
Q: Mass of oleic acid (glyceryl trioleate)=3.978g Mass of soap (sodium oleate)=3.8954g Moles of Oleic…
A:
Q: When balancing redox reaction in a basic solution, how many OH- should be added to each side?
A: In acidic medium, oxygen atoms are balanced by adding H2O molecules to the deficient side in O and…
Q: What are the solubility product expression and correct mathematical expressions for finding the…
A: In this question we have to write solubility product expression.
Q: What mass (in grams) of AgCl will dissolve in 250 mL of water if the Ksp = 1.7x10¬10? (MM of AgCl =…
A: Given:: Ksp of AgCl = 1.7×10-10 mass of AgCl = ? Volume = 250 ml = 0.250 L MM of AgCl = 143.32 g…
Q: Rank the following by increasing boiling points, so that 1 is the highest and 4 is the lowest.…
A: A question based on intermolecular forces that is to be accomplished.
Q: Complete the following nuclear equation and identify X. Potassium-40 ----> Beta + X
A: In this question we have to identify the X in the nuclear equation by balancing the nuclear…
Q: Questions 1. Provide the product(s) for each of the following reactions. a) NaOH b) CH,Br a) LDA b)…
A: If you are looking both reactions the given starting material ( cyclohexanone ) have equal alpha…
Q: Louie performed paper chromatography to identify which among the four available reference mixtures —…
A: Given: The distance travelled by the solvent from the origin is 79.0 mm. The distances (in cm)…
Q: Using the data given below, determine the relationship between Sstd and Cstd using an unweighted…
A:
Q: In a study of glass etching, a chemist examines the reaction between sand (SiO2) and hydrogen…
A:
Q: Calculate AG° for the electrochemical cell Fe(s) I Fe²*(aq) |I Snª*(aq) | Sn²"(aq) I Pt(s). 4+…
A:
Q: Which of the properties below apply to the following polymer? (CH (CH) Choose all that apply. a…
A: Given polymer structure is :
Q: Consider an ideal diatomic gas in a piston, at T = 22.7°C and atmospheric pressure (1.01 x 105 Pa)…
A:
Q: 6) Using the data table provided on the course website, determine the pH of a 0.631 M HCIOaq)…
A:
Q: n(s) + 2 OH"(aq) ZnO(s) + H20(e) + 2e 0:00 16:) + H20(8) + 2e¯ CC {e LH"( sider the animation…
A: Oxidising agent is the one which itself gets reduced Here HgO is an oxidising agent Because it is…
Q: What is the IUPAC name for the following
A: In this question we have to tell the IUPAC name of the compound.
Q: br Me A
A: In the given structure , there are two chiral center present in the given molecule . they give four…
Q: 1) LDA NH 1) NaOH ww XX 2) 2)H H 2) OE 3) H,O
A: Detail mechanistic pathway is given below
Q: 19.A sample consisting of 1 mol diatomic gas molecules at 200 K is compressed reversibly and…
A: Adiabatic Process : Thermodynamic process that occurs without transferring heat or mass between the…
Q: In this Chemical equation 2S2O32- (aq) + I2 (aq) —> S4O62- (aq) + 2I- (aq) with an ON of +2 -2 0…
A:
Q: 3. Complete the following reactions а. CH3 CH3-CH-CH-CH2-CH3 H2SO4 180°C ОН b. CH3 CH3 H2SO4…
A:
Q: What would the structure look like for the following: (CH3)2CFCBr3
A:
Q: The decomposition of HI at low temperature was studied by injecting 2.50 mol of HI into a 10.32-L…
A:
Q: Doc Jill wants to determine the Λ0 of a weak acid she isolated called Xcitingpartic acid (HXp). She…
A: Answer: When a weak acid will be added in water, its partial ionization takes place and due to…
Q: Prepare 250 mL of a stock solution that is 25 mg/L Fe, using the iron reagent ferrous ammonium…
A: To prepare 250 mL stock solution of Fe(NH4)2 (SO4)2•6H2O. Strength given = 25 mg/L = 0.025 g/L
Q: Ithough the equilibrium position changes as a result of a concentration or plume change, K does not.…
A: In this question, we will select the true / false for the given statement. You can see the details…
Q: Balance the following oxidation-reduction reaction that occurs in an acidic solution using the…
A: Given Chemical reaction: I- (aq)+ ClO- (aq) → I3- (aq)+ Cl- (aq) Step 1. Write the oxidation -…
Q: Which of these can be rinsed down the sink? the left over liquid from a distillation of 1-butanol…
A: The correct answer is Option (4) None of them should go down the sink.
Q: Full Mechanism 4 4-dimethyl-2 5-cyclohexadien-1-one + HCl ----> 3 4-dimethylcyclohexanol
A:
Q: 20.77. The nitration of methanol, CH;OH, by nitrous acid is thought to proceed via the following…
A:
Q: BaSO, precipitated from a 1.800 g sample was contaminated with 9.4 mg of Ferrous sulfate and weighed…
A:
Q: 0.185 M in HCHO2 (Ka = 1.8 × 10-4) and 0.230 M in HC2H3O2 (Ka = 1.8 × 10¬5) Express your answer to…
A: Answer: In this question we have to find out the pH of the solution of two weak acids. In the…
Q: HO- 1. Hg(OAc)2, 2. NaBH4 NaOH Br HO,
A:
Q: Which of the following structures is the cis configuration of 1,3-dimethylcyclohexane with both…
A: Cyclohexene exist in two confirmation: chair and boat confirmation. Chair confirmation is more…
Q: Provide a detailed electron pushing mechanism describing the following conversion HCI Меон 'N. Br2…
A:
Q: following reaction is the only that occurs, will the Cu react completely? Cu(s) + HNO,(aq)<→…
A: According to the given chemical reaction, 1 mole Cu react with 1 mole of HNO3…
Q: Gravimetric analysis. Please show complete solution. Thank you Question: A 3.00- g sample of an…
A:
Q: 5. Predict the geometries of NF, using the VSSPR method with the following steps Step 1) Draw the…
A: The ammonium cation is a positively charged polyatomic ion with the chemical formula NH4+. It is…
Q: duct of the reduction of Cr,0, (ag)? Can Fe(s) reduce Sn (ag) to Sn (ag)? If yes, what is the…
A: Reaction 1: In the chemical reaction, Cr2O7^(-2) oxidizes Fe2+ to Fe3+. The chemical reaction is…
Q: Referring to the named structures below, Choose: A if 1 st statement is true and the second…
A: Hydrocarbons are compounds made up of only carbon and hydrogen; can be classified in to two main…
Q: ्छुकण्ट नैनंजपर जपपट ज मह पडरव्ंट ककर कीववे ड oocंबटd कटक खकं नी कhर कीठिमेकम तनतरिण्पकव रठ…
A: Sodium ethoxide is base and it will abstract acidic proton from active methylene group which…
Q: Give the clear handwritten answer with explanation...give the mechanism of sub parts given bleow…
A:
Q: candle wax burned in the experiment. 9. Calculate What is the heat of combustion of candle wax in…
A:
Q: Experiment 3. Assay of Cupric Sulfate Principle: lodometry The percentage purity of cupric sulfate…
A:
Q: 2. Consider the reaction: NH Ga) + 2NO2 (aq) → N2) + 2H2O Rate = k[NH4*[NO2] Which of the following…
A: Rate of reaction :- The increase in concentration products with time or decrease in concentration…
Q: Draw the Condensed and Line Angle structural formula of the IUPAC name given. 3 - ethylheptane -…
A: The naming of an organic compound can be done with help of rules of international union of pure and…
Q: Which of the monomers shown forms the addition polymer polyethylene ? H. H F Он H. CN H.
A: addition polymerization: The polymerization reaction which involves joining of unsaturated monomer…
Q: What is the normality of the new solution if a 200.0 mL of a 0.40 M HCl solution is diluted with…
A: Given: Initial volume of HCl solution= 200.0 mL Initial molarity of HCl solution= 0.40 M Final…
Q: In the protection of functional groups in the synthesis of ibuprofen, the acid group is converted to…
A: Functional groups are the species attached to carbon chain and which gives a unique identity to the…
Step by step
Solved in 3 steps

- In the standardi zation of HCl using pure anhydrou s sodium carbonate as primarystandard for methy l orange as indica tor , 1.0 mL HCl was found to be equiva lent to 0.05gof sodium carbonate (MW =106). The no rmality of HCl isHexanoic acid was added to an immiscible biphasic solvent sysem, water and CCl4 at 20.0OC and the equilibrium concentrations of hexanoic acid were determined to be 3.66 g/L in H2O and 67.0 g/L in CCl4. Caluclate the distrubution coeffiecent (D1) of hexanoic acid in CCl4 with respect to water.A 0.1 M solution of acid was used to titrate 10 ml of 0.1 M solution of alkali and thefollowing volumes of acid were recorded: 9.88 10.18 10.23 10.39 10.21 Calculate the 95% confidence limits of the mean and use them to decide whetherthere is any evidence of systematic error.
- It is required to know the concentration of an aqueous solution of H2SO4 that appeared in the laboratoryChemistry III and it's unlabeled. To this end, a student of analytical chemistry carried out the followingProcedure: He took 5.00 mL of a fresh and standardized solution of 0.525M NaOH and brought them to a250.0 mL balloon to be completed with distilled water. Subsequently, he poured 15.00 mL of the solutionH2SO4 of unknown concentration in an Erlenmeyer flask and added 2 drops of phenolphthalein.Using a burette filled with the last NaOH solution, he noticed that when adding 39.40 mL of the hydroxidethe Erlenmeyer solution reached a faint but permanent pink. With the above dataDetermine the concentration and pH of the H2SO4 solution.A 370.00 mL solution of 0.00190 M AB5 is added to a 200.00 mL solution of 0.00165 M CD2. What is pQsp for AD5?How to prepare 250cm3 of a solution of carbonate with concentration 0.100 mol dm-3
- Calculate the mole fractions of SO2•H2O, HSO3- and SO3 2- in solution at a pH of 3.0, characteristic of many fogs, as well as the total concentration of S(IV) in solution for 20 ppb SO2 in the gas phase. Constants attached in tableYou are supplied with the following: / Jy word voorsien van die volgende: NaCl(Mr= 58,443 g /mol) 2.5MTris-Cl, pH 8 solution /oplossing (1 Litre) EDTA,natriumsout(Mr= 380,2g/mol) 10% sodium dodecyl sulphate solution / natriumdodecyl sulfaat oplossing Proteïnase K solution / oplossing (50 mg dissolved / opgelos in 1 ml ddH2O) You need a digestion buffer consisting of the following: / Jy moet 'n verteringsbuffer op maak wat uit die volgende bestaan: 15m M NaCl 75 mM Tris-Cl,pH 8 16 mM EDTA,pH 8 0.8% sodium sulphate / natrium dodecyl sulfaat 0,75 mg/ml proteïnase K How will you prepare 500 ml of the digestion buffer? Show all your steps and calculations. Remember to explain exactly how you will make it up.but my prof said the answer is H2SO4,NaCN and H2O for solvent. why did he answer like above? and please tell me how did u find KCN and HCN what makes you answer like that Should i have to memorize some solvent or reagent? then let me kno that list
- Q1. Dissolved 0.273 grams of pure sodium oxalate (Na2C0.) In distilled water and added sulfuric acid and titration the solution at 70 ° C by using 42.68 ml of KMnO, solution and has exceeded end point limits by using 1.46 ml of oxalic acid (H , C, 0.) With 0.1024 N. Calculate the normlity of KMnO .. Note that the molecular weight of sodium oxalate (Na, C, 0.) = 134 and its equivalent weight = 67. From the journal entitled “Ionic liquids synthesis and applications: An overview”,written by Sandip K. Singh and Anthony W. Savoy (attached with this assignment),Elucidate the:(a) SynthesisPrepare 0.1 M solutions of NaOH and 0.1 M ethyl acetate using high-purity distilled water. So, weight desired amount of NaOH and ethyl acetate and dissolved in dH2O to prepare stock solution in equal molarity. Mw (NaOH) = 40.0 g/mol , Mw (EtOAc)= 88.1 g/mol, Density(EtOAc): 0.898 g/cm3



