Using the data given below, determine the relationship between Sstd and Cstd using an unweighted linear regression. Cstd (arbitrary units) Sstd(arbitrary units) KA= Sstd C std 0.000 0.00 0.100 12.36 123.6 0.200 24.83 124.2 0.300 35.91 119.7 0.400 48.79 122.0 0.500 60.42 122.8 Calculate the 95% confidence intervals for the slope and y-intercept.
Using the data given below, determine the relationship between Sstd and Cstd using an unweighted linear regression. Cstd (arbitrary units) Sstd(arbitrary units) KA= Sstd C std 0.000 0.00 0.100 12.36 123.6 0.200 24.83 124.2 0.300 35.91 119.7 0.400 48.79 122.0 0.500 60.42 122.8 Calculate the 95% confidence intervals for the slope and y-intercept.
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 8P
Related questions
Question
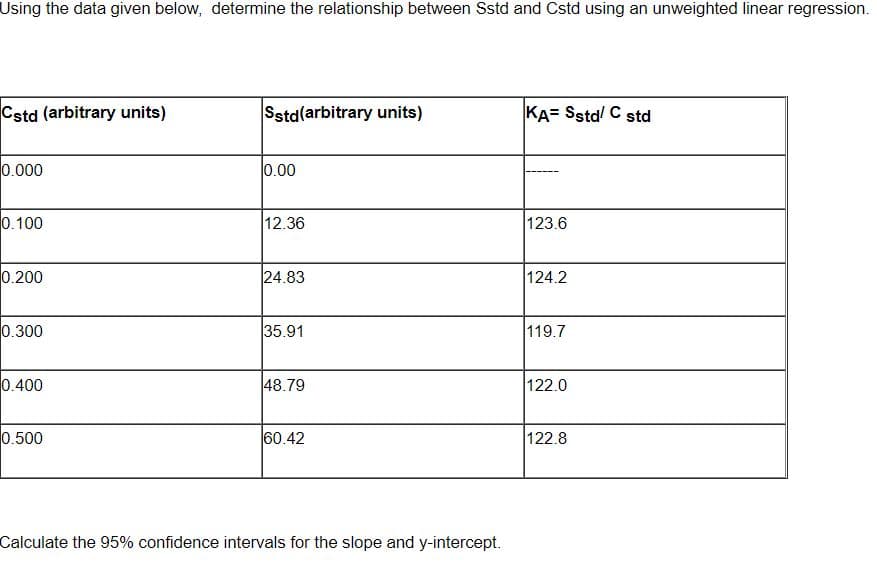
Transcribed Image Text:Using the data given below, determine the relationship between Sstd and Cstd using an unweighted linear regression.
Cstd (arbitrary units)
Sstd(arbitrary units)
KA= Sstd! C std
0.000
0.00
0.100
12.36
123.6
0.200
24.83
124.2
0.300
35.91
119.7
0.400
48.79
122.0
0.500
60.42
122.8
Calculate the 95% confidence intervals for the slope and y-intercept.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



