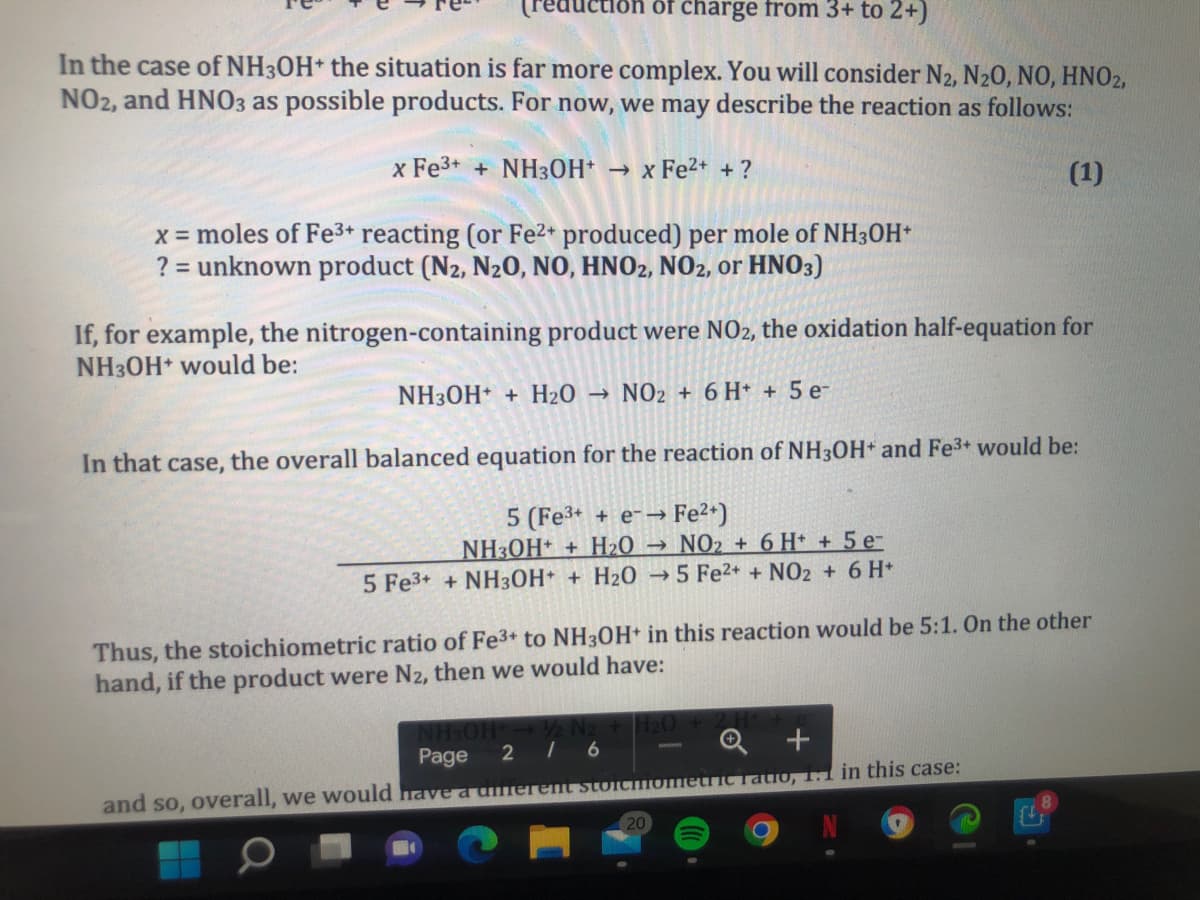
In the case of NH30H+ the situation is far more complex. You will consider N2, N20, NO, HNO2, NO2, and HNO3 as possible products. For now, we may describe the reaction as follows: x Fe3+ + NH3OH* x Fe2+ +? (1) x = moles of Fe3+ reacting (or Fe2+ produced) per mole of NH3OH* ? = unknown product (N2, N20, NO, HNO2, NO2, or HNO3) If, for example, the nitrogen-containing product were NO2, the oxidation half-equation for NH30H would be: NH30H + H20 → NO2 + 6 H* + 5 e- In that case, the overall balanced equation for the reaction of NH3OH and Fe3+ would be: 5 (Fe3+ + e- Fe2*) NH3OH* + H2O → NO2 + 6 H* + 5e- 5 Fe3+ + NH30H+ + H2O →5 Fe2+ + NO2 + 6 H* Thus, the stoichiometric ratio of Fe3+ to NH30H in this reaction would be 5:1. On the other hand, if the product were N2, then we would have: | 6 Q + Page 2 and so, overall, we would havea dferemt stoiciometric Tatio, 1.1 in this case:
In the case of NH30H+ the situation is far more complex. You will consider N2, N20, NO, HNO2, NO2, and HNO3 as possible products. For now, we may describe the reaction as follows: x Fe3+ + NH3OH* x Fe2+ +? (1) x = moles of Fe3+ reacting (or Fe2+ produced) per mole of NH3OH* ? = unknown product (N2, N20, NO, HNO2, NO2, or HNO3) If, for example, the nitrogen-containing product were NO2, the oxidation half-equation for NH30H would be: NH30H + H20 → NO2 + 6 H* + 5 e- In that case, the overall balanced equation for the reaction of NH3OH and Fe3+ would be: 5 (Fe3+ + e- Fe2*) NH3OH* + H2O → NO2 + 6 H* + 5e- 5 Fe3+ + NH30H+ + H2O →5 Fe2+ + NO2 + 6 H* Thus, the stoichiometric ratio of Fe3+ to NH30H in this reaction would be 5:1. On the other hand, if the product were N2, then we would have: | 6 Q + Page 2 and so, overall, we would havea dferemt stoiciometric Tatio, 1.1 in this case:
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 26Q: How can one construct a galvanic cell from two substances, each having a negative standard reduction...
Related questions
Question
100%
For each possible product, determine x in equation 1
(Equation 1 and oxidation states for each possible product found in attached images)

Transcribed Image Text:N+4-2=+1
2) Ondation state of N in NHs OH": -1
Onidahion stale of N in:
N20
N2
NO
HNO2
NO2
HNO,
+2/2
-2
-2
H +3 -2(2):-4
-2(2):4
H +5 -2(3):-6
+2
+4
+2
+3
+5
ht4
Transcribed Image Text:reduc
of charge from 3+ to 2+)
In the case of NH3OH* the situation is far more complex. You will consider N2, N20, NO, HNO2,
NO2, and HNO3 as possible products. For now, we may describe the reaction as follows:
x Fe3+ + NH3OH* → x Fe²* + ?
(1)
x = moles of Fe3+ reacting (or Fe2* produced) per mole of NH3OH*
? = unknown product (N2, N20, NO, HNO2, NO2, or HNO3)
If, for example, the nitrogen-containing product were NO2, the oxidation half-equation for
NH3OH would be:
NH30H + H20
NO2 + 6 H* + 5 e-
In that case, the overall balanced equation for the reaction of NH3OH* and Fe3+ would be:
> Fe2+)
NH3OH* + H2O → NO2 + 6 H* + 5 e-
5 Fe3+ + NH3OH+ + H2O → 5 Fe2+ + NO2 + 6 H*
5 (Fe3+ + e-→
Thus, the stoichiometric ratio of Fe3+ to NH30H+ in this reaction would be 5:1. On the other
hand, if the product were N2, then we would have:
2 I 6
Q +
Page
|3D
Tatio, 1.1 1in this case:
and so, overall, we would have a umerent stoicfometi
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning