In the ionization reaction of pyridine, C5H5N (aq) + H₂O (1) = C5H5NH+ (aq) + OH¯ (aq) the equilibrium concentrations of pyridine, C₂HÃN, pyridinium, C5H5NH+, and hydroxide OH, are 2.00 M, 5.83 × 10-5 M, and 5.83 × 10-5 M, respectively. Calculate the value of K. 1.70 × 10-⁹ 5.83 x 10-5 5.88 × 108 5.00 x 10-1
In the ionization reaction of pyridine, C5H5N (aq) + H₂O (1) = C5H5NH+ (aq) + OH¯ (aq) the equilibrium concentrations of pyridine, C₂HÃN, pyridinium, C5H5NH+, and hydroxide OH, are 2.00 M, 5.83 × 10-5 M, and 5.83 × 10-5 M, respectively. Calculate the value of K. 1.70 × 10-⁹ 5.83 x 10-5 5.88 × 108 5.00 x 10-1
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section: Chapter Questions
Problem 44PS
Related questions
Question
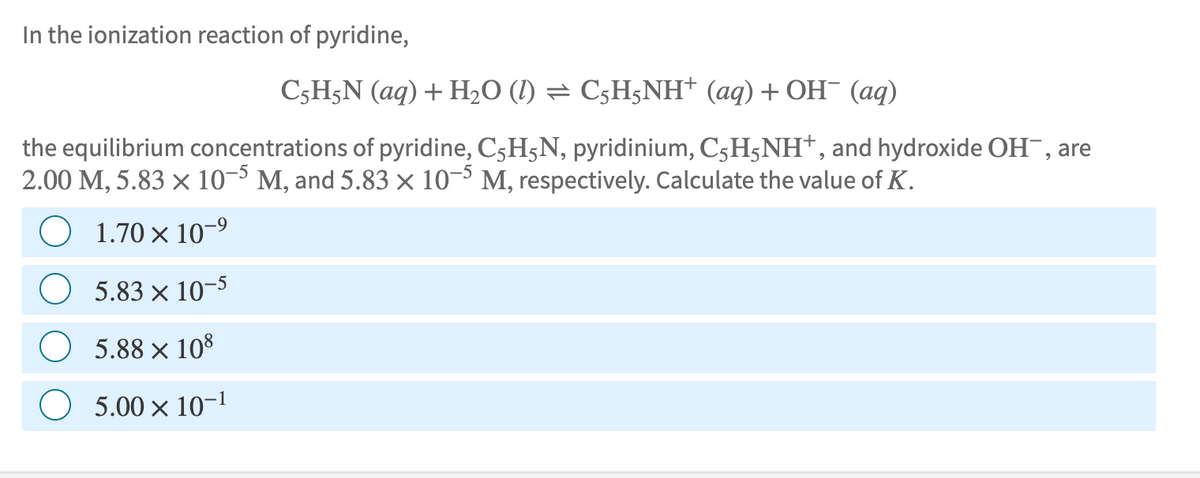
Transcribed Image Text:In the ionization reaction of pyridine,
C5H5N (aq) + H₂O (1) = C5H5NH+ (aq) + OH¯ (aq)
the equilibrium concentrations of pyridine, C₂HÃN, pyridinium, C5H5NH+, and hydroxide OH, are
2.00 M, 5.83 × 10-5 M, and 5.83 × 10-5 M, respectively. Calculate the value of K.
1.70 × 10-⁹
5.83 x 10-5
5.88 × 108
5.00 x 10-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax