In the lungs, the respiratory membrane separates tiny sacs of air (pressure 1.00x10°Pa) from the blood in the capillaries. These sacs are called alveoli. The average radius of the alveoli is 0.125 mm, and the air inside contains 14% oxygen. Assuming that the air behaves as an ideal gas at 310K, calculate the number of oxygen molecules in one of these sacs. The pressure of an ideal gas is reduced by 50%, resulting in a decrease in temperature to 75% of the initial value. Calculate the ratio of the final to initial volumes of the gas.
In the lungs, the respiratory membrane separates tiny sacs of air (pressure 1.00x10°Pa) from the blood in the capillaries. These sacs are called alveoli. The average radius of the alveoli is 0.125 mm, and the air inside contains 14% oxygen. Assuming that the air behaves as an ideal gas at 310K, calculate the number of oxygen molecules in one of these sacs. The pressure of an ideal gas is reduced by 50%, resulting in a decrease in temperature to 75% of the initial value. Calculate the ratio of the final to initial volumes of the gas.
University Physics Volume 1
18th Edition
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:William Moebs, Samuel J. Ling, Jeff Sanny
Chapter1: Units And Measurement
Section: Chapter Questions
Problem 87AP: A car engine moves a piston with a circular cross-section of 73000.002cm in diameter a distance of...
Related questions
Question
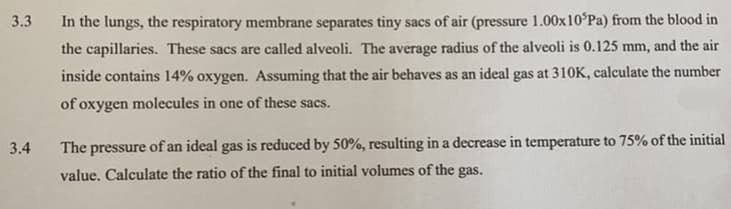
Transcribed Image Text:3.3
In the lungs, the respiratory membrane separates tiny sacs of air (pressure 1.00x10°Pa) from the blood in
the capillaries. These sacs are called alveoli. The average radius of the alveoli is 0.125 mm, and the air
inside contains 14% oxygen. Assuming that the air behaves as an ideal gas at 310K, calculate the number
of oxygen molecules in one of these sacs.
3.4
The pressure of an ideal gas is reduced by 50%, resulting in a decrease in temperature to 75% of the initial
value. Calculate the ratio of the final to initial volumes of the gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College