In the methane molecule, CH4, each hydrogen atom is at the corner of a regular tetrahedron with the carbon atom at the center. If one of the C-H is in the direction of A= î + î +R and an adjacent C-H bond is at the direction B=î-Î-R. results to an angular bond of approximately 109° for a static frozen molecule. However, the molecule we can encounter everyday continuously vibrates and interact with the surrounding causing its bond vector to vary slightly. According to a new spectroscopy analysis, the adjacent bond vectors was found to be A = 1.02i + 0.87) + 0.96k B = 0.94i + -1.09j + -0.92k What is the angle (in degrees) between the bonds based on this new data?
In the methane molecule, CH4, each hydrogen atom is at the corner of a regular tetrahedron with the carbon atom at the center. If one of the C-H is in the direction of A= î + î +R and an adjacent C-H bond is at the direction B=î-Î-R. results to an angular bond of approximately 109° for a static frozen molecule. However, the molecule we can encounter everyday continuously vibrates and interact with the surrounding causing its bond vector to vary slightly. According to a new spectroscopy analysis, the adjacent bond vectors was found to be A = 1.02i + 0.87) + 0.96k B = 0.94i + -1.09j + -0.92k What is the angle (in degrees) between the bonds based on this new data?
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter12: Oscillatory Motion
Section: Chapter Questions
Problem 64P: A smaller disk of radius r and mass m is attached rigidly to the face of a second larger disk of...
Related questions
Question
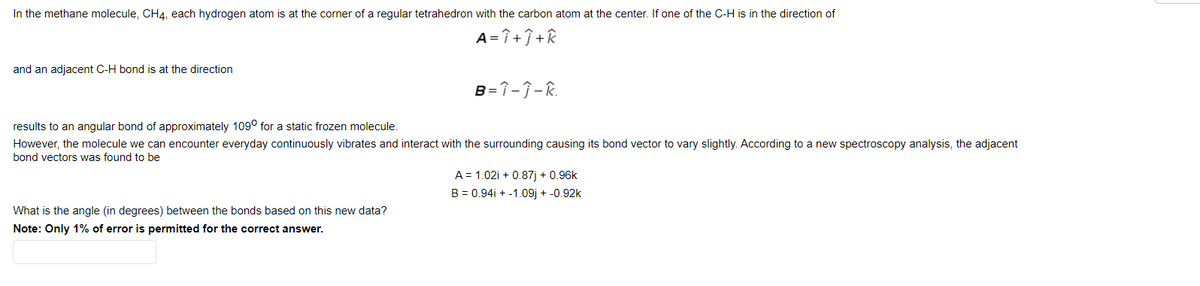
Transcribed Image Text:In the methane molecule, CH4, each hydrogen atom is at the corner of a regular tetrahedron with the carbon atom at the center. If one of the C-H is in the direction of
A=î+î +k
and an adjacent C-H bond is at the direction
B=î-ĵ-R.
results to an angular bond of approximately 109° for a static frozen molecule.
However, the molecule we can encounter everyday continuously vibrates and interact with the surrounding causing its bond vector to vary slightly. According to a new spectroscopy analysis, the adjacent
bond vectors was found to be
A = 1.02i + 0.87j + 0.96k
B = 0.94i + -1.09j + -0.92k
What is the angle (in degrees) between the bonds based on this new data?
Note: Only 1% of error is permitted for the correct answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning