In the Periodic Table below, shade all the elements for which the neutral atom has an outer electron configuration of ms'nd", where n and m are integers, and m=n+1. H He Li Be BCNOF Ne Na Mg Al Si PS CI Ar K Ca Sc Ti VCr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y | Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta WRe Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn
In the Periodic Table below, shade all the elements for which the neutral atom has an outer electron configuration of ms'nd", where n and m are integers, and m=n+1. H He Li Be BCNOF Ne Na Mg Al Si PS CI Ar K Ca Sc Ti VCr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y | Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta WRe Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 64GQ: Answer the questions below concerning ground state electron configurations. (a) What element has the...
Related questions
Question
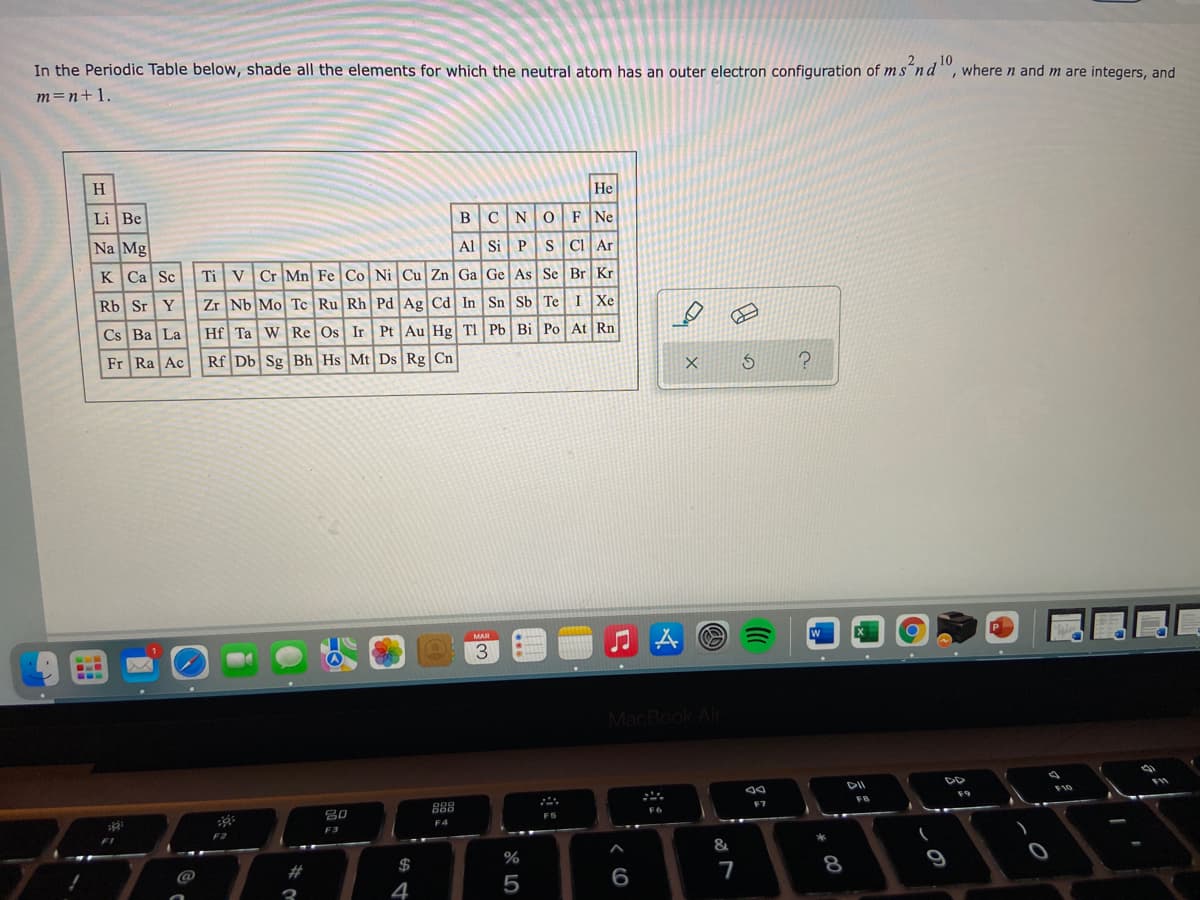
Transcribed Image Text:10
In the Periodic Table below, shade all the elements for which the neutral atom has an outer electron configuration of ms´nd", where n and m are integers, and
m=n+1.
H
Не
Li Be
BCNO F Ne
Na Mg
Al Si PS C Ar
K Ca Sc
Ti VCr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La
Hf Ta WRe Os Ir Pt Au Hg TI Pb Bi Po At Rn
Fr Ra Ac
Rf Db Sg Bh Hs Mt Ds Rg Cn
MAR
3.
673
MacBook Air
FB
F7
F5
F4
F3
&
%
$
8
2#
3
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning