Consider three molecules, H79Br and deuterated molecule D79B and H$1 Br. The lowest energy transition for H79Br is 8.48572 cm-1. Compute the bond length of H79Br. Next compute the change in bond length by deuteration. Finally, briefly comment on how isotopic exchange changes both vibrational and rotational spectra. How are these changes similar, how do they differ? Is the exchange of H or Br more important for each case?
Consider three molecules, H79Br and deuterated molecule D79B and H$1 Br. The lowest energy transition for H79Br is 8.48572 cm-1. Compute the bond length of H79Br. Next compute the change in bond length by deuteration. Finally, briefly comment on how isotopic exchange changes both vibrational and rotational spectra. How are these changes similar, how do they differ? Is the exchange of H or Br more important for each case?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 62P
Related questions
Question
P1
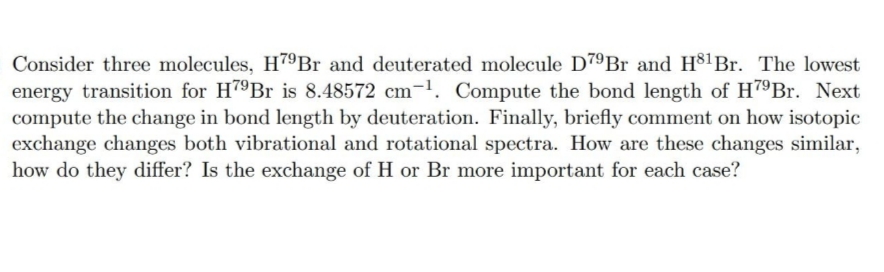
Transcribed Image Text:Consider three molecules, H79Br and deuterated molecule D7ºBr and H$1Br. The lowest
energy transition for H79B is 8.48572 cm-1. Compute the bond length of H79Br. Next
compute the change in bond length by deuteration. Finally, briefly comment on how isotopic
exchange changes both vibrational and rotational spectra. How are these changes similar,
how do they differ? Is the exchange of H or Br more important for each case?
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,