In this task, various ideal gases are considered. If no other information is given, the specific gas constant R = 252.6 J/(kg K). Unless otherwise stated, all changes of state dbe regarded as quasi-static and take place in a closed system. All parts of the task dbe solved independently of each other. Indices indicate the initial state (index 1) and the final state (index 2). The general gas constant Rm = 8.314462 J/(mol K) applies to the entire task. Hint: - Round the results to three decimal places (e.g. 58.189 or 0.015). a) An ideal gas with p = 1907.6 mbar and T = 270.1 K is in a container with a volume of V = 720 cm3 . What quantity of substance (in mol) is in the container? What is the pressure p2 (in kPa) after an isothermal change of state (T = 310.2 K), in which a specific volume of vi = 0.12 m3/kg is initially present and |912 |= 101 kJ/kg specific heat is dissipated? b) c) Three ideal gases were mixed in a closed container. The mixture has a pressure of p = 1.5 bar, the mole fraction of component 1 isi = 0.1 and the partial pressure of component 3 is ps = 0.825 bar. What valibe the pressure (in bar) that would prevail if component 2 occupied the entire container alone (components 1 and 3 wereremoved from the container)?
In this task, various ideal gases are considered. If no other information is given, the specific gas constant R = 252.6 J/(kg K). Unless otherwise stated, all changes of state dbe regarded as quasi-static and take place in a closed system. All parts of the task dbe solved independently of each other. Indices indicate the initial state (index 1) and the final state (index 2). The general gas constant Rm = 8.314462 J/(mol K) applies to the entire task. Hint: - Round the results to three decimal places (e.g. 58.189 or 0.015). a) An ideal gas with p = 1907.6 mbar and T = 270.1 K is in a container with a volume of V = 720 cm3 . What quantity of substance (in mol) is in the container? What is the pressure p2 (in kPa) after an isothermal change of state (T = 310.2 K), in which a specific volume of vi = 0.12 m3/kg is initially present and |912 |= 101 kJ/kg specific heat is dissipated? b) c) Three ideal gases were mixed in a closed container. The mixture has a pressure of p = 1.5 bar, the mole fraction of component 1 isi = 0.1 and the partial pressure of component 3 is ps = 0.825 bar. What valibe the pressure (in bar) that would prevail if component 2 occupied the entire container alone (components 1 and 3 wereremoved from the container)?
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Please try to solve complete in one hour

Transcribed Image Text:In this task, various ideal gases are considered. If no other information is given, the specific gas
constant R = 252.6 J/(kg K). Unless otherwise stated, all changes of state ebe regarded as
quasi-static and take place in a closed system. All parts of the task dbe solved independently of
each other. Indices indicate the initial state (index 1) and the final state (index 2). The
general gas constant Rm = 8.314462 J/(mol K) applies to the entire task.
Hint:
- Round the results to three decimal places (e.g. 58.189 or 0.015).
a)
An ideal gas with p = 1907.6 mbar and T = 270.1 K is in a container with a
volume of V = 720 cm3 . What quantity of substance (in mol) is in the
container?
b)
What is the pressure p2 (in kPa) after an isothermal change of state (T =
310.2 K), in which a specific volume of vi = 0.12 m3/kg is initially present and
|912 | = 101 kJ/kg specific heat is dissipated?
c)
Three ideal gases were mixed in a closed container. The mixture has a
pressure of p = 1.5 bar, the mole fraction of component 1 isı = 0.1 and the
partial pressure of component 3 is p3 = 0.825 bar. What vakabe the pressure
(in bar) that would prevail if component 2 occupied the entire container
alone (components 1 and 3 wereremoved from the container)?
d)
An ideal gas (Ti = 540 K, vı = 2.1 m3/kg,
= 1.5) isadiabatically and reversibly
compressed to a pressure of p2 = 400 kPa. What is then the specific volume
(in m3/kg)?
e)
A dgmedium is to be selected for the design of a heat exchanger with adiabatic
external walls. Since the installation space is limited, the mass flow of the
cold gas is only one tenth of the warm mass flow. Nevertheless, the warm
mass flow is to be cooled by 101 K, while the cold mass flow is heated by 45
K. The mass flow of the cold gas is to be cooled by 45 K. What must be the
ratio of the isobaric labapacities Cp,kalt /Cp,warm ?
f)
Two stationary flow processes are coupled and 91% of the technical work
output of a reversible isochoric pressure reduction from pi = 4.1 MPa to
p = 3.5 MPa at vi = 0.1 m3/kg is added to an isen- tropic compression (3 !
4). What is the pressure ratio p4 /p ? 3
achieved during isentropic compression (t3 = 21142 ac, = 1.28)?
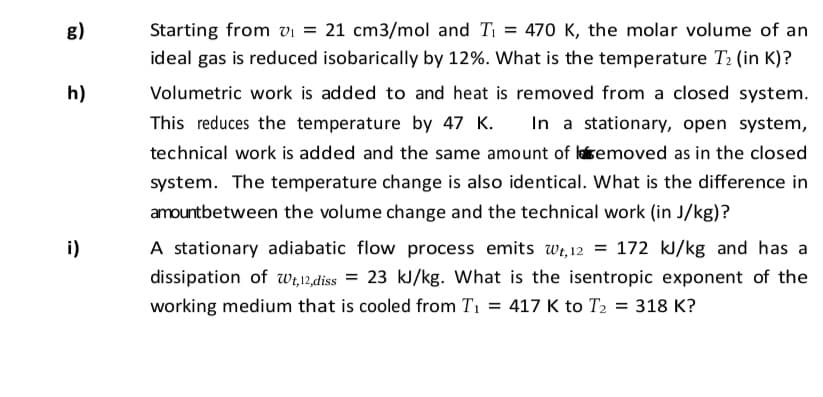
Transcribed Image Text:g)
Starting from vi = 21 cm3/mol and Ti = 470 K, the molar volume of an
ideal gas is reduced isobarically by 12%. What is the temperature T2 (in K)?
h)
Volumetric work is added to and heat is removed from a closed system.
This reduces the temperature by 47 K.
In a stationary, open system,
technical work is added and the same amount of laisemoved as in the closed
system. The temperature change is also identical. What is the difference in
amountbetween the volume change and the technical work (in J/kg)?
i)
A stationary adiabatic flow process emits wi, 12 = 172 kJ/kg and has a
dissipation of wi,12,diss = 23 kJ/kg. What is the isentropic exponent of the
working medium that is cooled from T1 = 417 K to T2 = 318 K?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY