In titration of 35 ml 0.046 M Fe2* with 0.056 M Ce4* what is the cell voltage at the equivalence point? E(calomel) = 0.241 V Fe3+ + e¯ 2 Fe²+ E° = 0.767 V Ce4+ + e- = Ce3+ E° = 1.70 V Buret containing Ce Calomel
In titration of 35 ml 0.046 M Fe2* with 0.056 M Ce4* what is the cell voltage at the equivalence point? E(calomel) = 0.241 V Fe3+ + e¯ 2 Fe²+ E° = 0.767 V Ce4+ + e- = Ce3+ E° = 1.70 V Buret containing Ce Calomel
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.29QAP
Related questions
Question
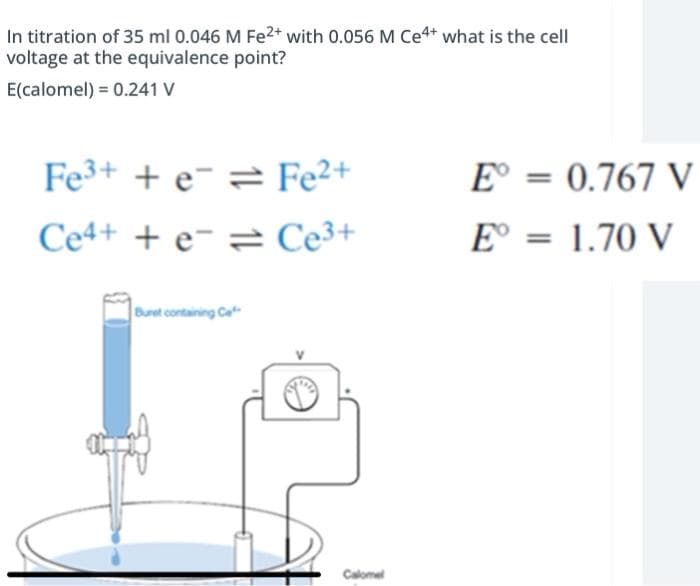
Transcribed Image Text:In titration of 35 ml 0.046 M Fe2* with 0.056 M Ce4* what is the cell
voltage at the equivalence point?
E(calomel) = 0.241 V
Fe3+ + e¯ = Fe²+
E° = 0.767 V
Ce4+ + e- = Ce3+
E° = 1.70 V
Buret containing Ce
Calomel
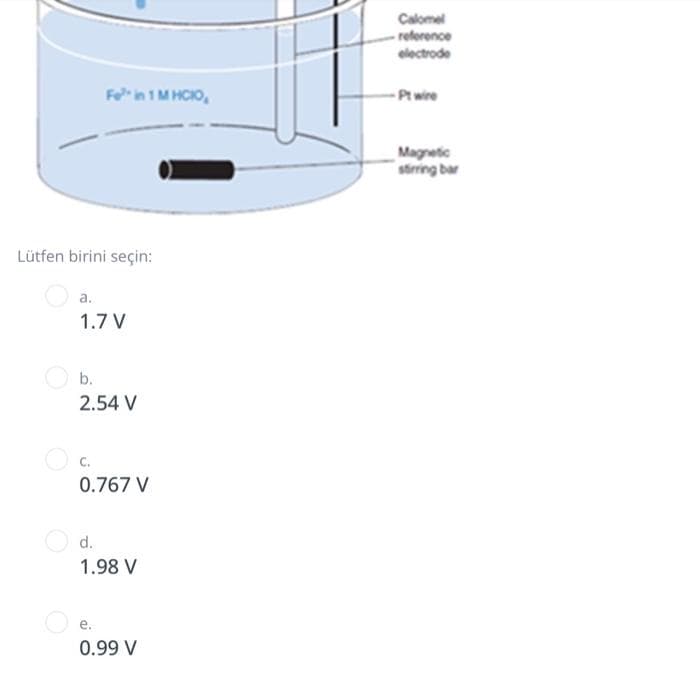
Transcribed Image Text:Calomel
- reforence
electrode
Fe in 1 M HCIO,
-Pt wire
Magnetic
string bar
Lütfen birini seçin:
а.
1.7 V
b.
2.54 V
0.767 V
d.
1.98 V
O e.
0.99 V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



