7. Reactions A and B were studied and found to produce the given energy diagrams. 150 150 Energy 100 (kJ/mol) Energy 100 (kJ/mol) 50 50 Reaction progress Reaction progress Reaction A Reaction B A shird reaction, C was studied with a measured activation energy of 55 KJ/mol. Assume all reactions take place at the same temperature. a. Reaction A is slowest b. Reaction B is slowest c. Reaction C is slowest d. All reactions happen at the same rate.
7. Reactions A and B were studied and found to produce the given energy diagrams. 150 150 Energy 100 (kJ/mol) Energy 100 (kJ/mol) 50 50 Reaction progress Reaction progress Reaction A Reaction B A shird reaction, C was studied with a measured activation energy of 55 KJ/mol. Assume all reactions take place at the same temperature. a. Reaction A is slowest b. Reaction B is slowest c. Reaction C is slowest d. All reactions happen at the same rate.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter13: Rates Of Reaction
Section: Chapter Questions
Problem 13.126QP
Related questions
Question
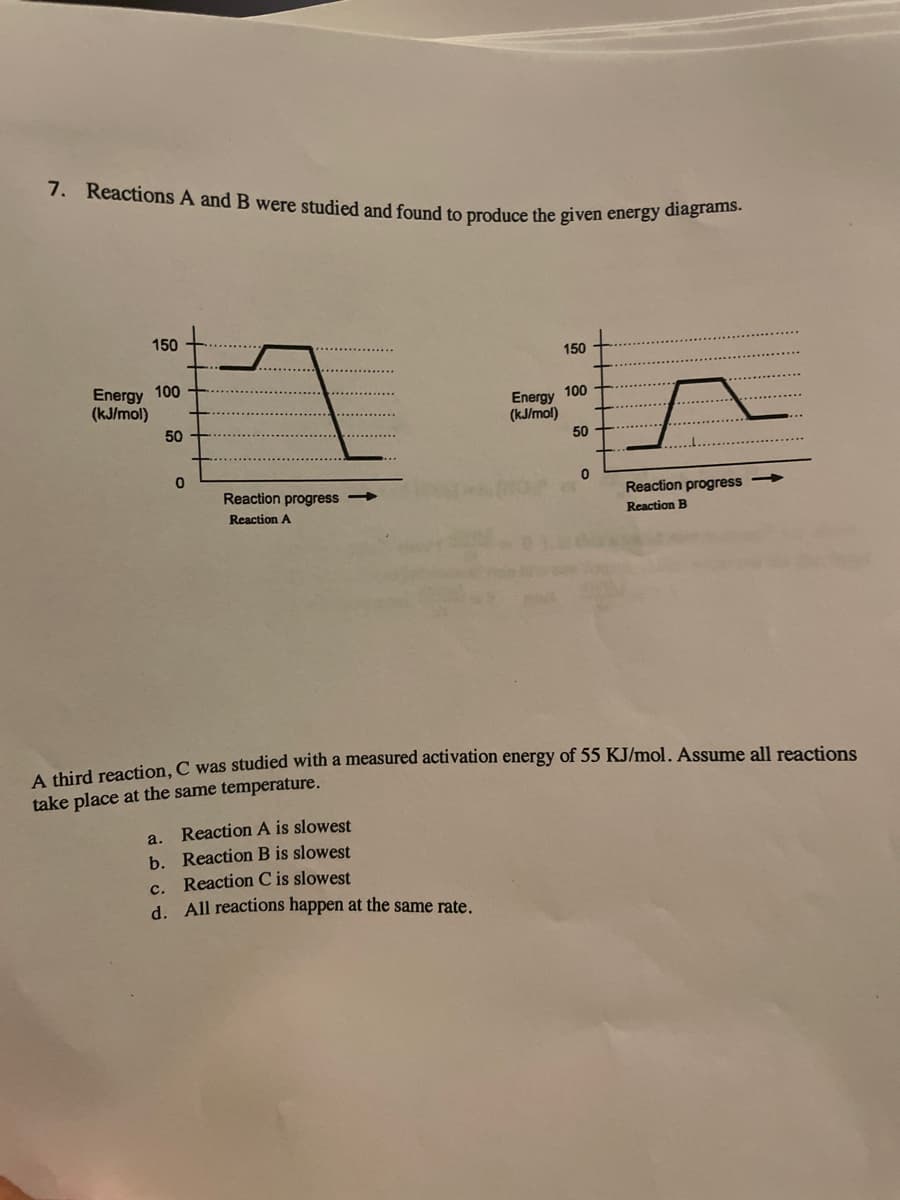
Transcribed Image Text:7. Reactions A and B were studied and found to produce the given energy diagrams.
150
150
Energy 100
(kJ/mol)
100
Energy
(kJ/mol)
50
50
Reaction progress
Reaction progress
Reaction A
Reaction B
A third reaction, C was studied with a measured activation energy of 55 KJ/mol. Assume all reactions
take place at the same temperature.
Reaction A is slowest
a.
b. Reaction B is slowest
c. Reaction C is slowest
d. All reactions happen at the same rate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning