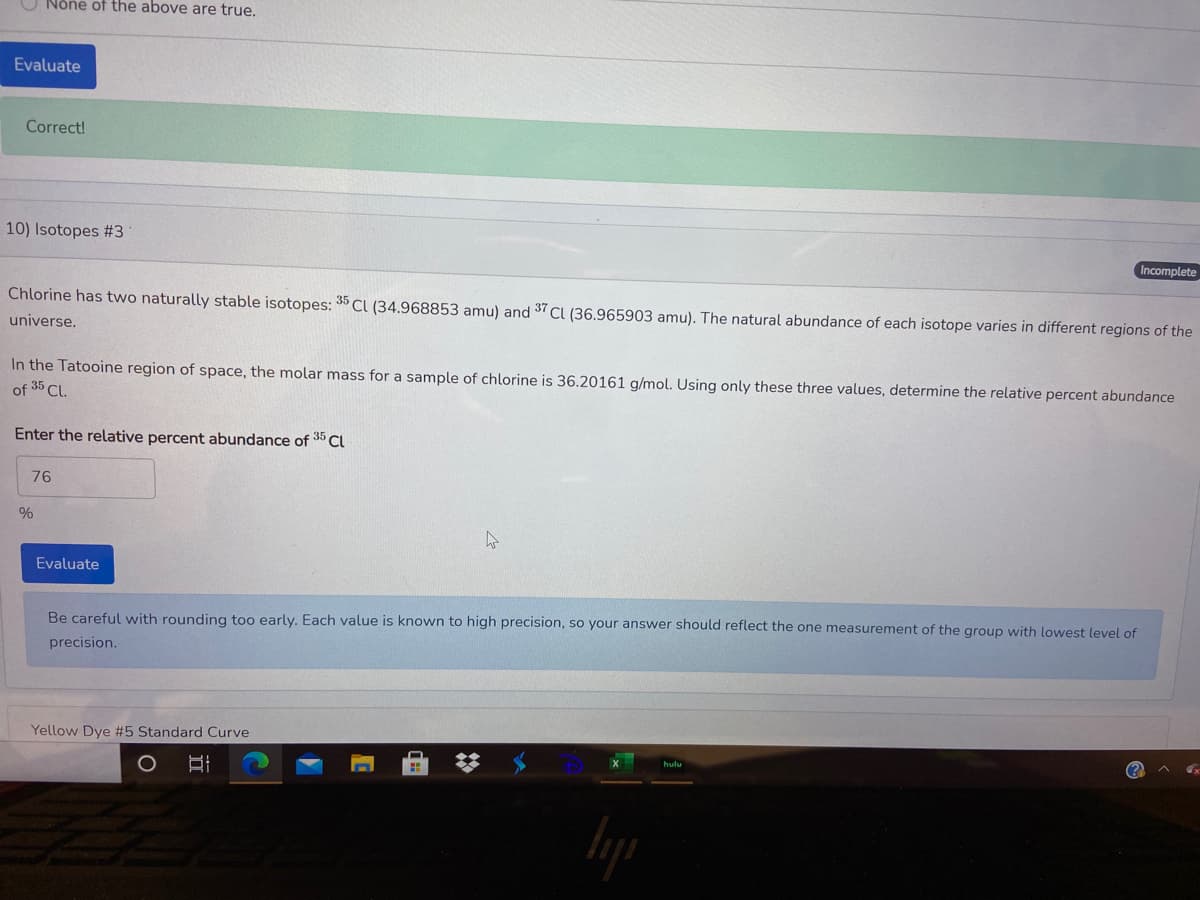
Incomplete Chlorine has two naturally stable isotopes: 35 CI (34.968853 amu) and 37 CI (36.965903 amu). The natural abundance of each isotope varies in different regions of the universe. In the Tatooine region of space, the molar mass for a sample of chlorine is 36.20161 g/mol. Using only these three values, determine the relative percent abundance of 35 CL. Enter the relative percent abundance of 35 Cl 76
Incomplete Chlorine has two naturally stable isotopes: 35 CI (34.968853 amu) and 37 CI (36.965903 amu). The natural abundance of each isotope varies in different regions of the universe. In the Tatooine region of space, the molar mass for a sample of chlorine is 36.20161 g/mol. Using only these three values, determine the relative percent abundance of 35 CL. Enter the relative percent abundance of 35 Cl 76
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section2.2: Isotopes And Atomic Weight
Problem 2.3CYU: Neon has three stable isotopes, one with a small abundance. What are the abundances of the other two...
Related questions
Question
Transcribed Image Text:Nôñe of the above are true.
Evaluate
Correct!
10) Isotopes #3
Incomplete
Chlorine has two naturally stable isotopes: 35 CI (34.968853 amu) and 37 CI (36.965903 amu). The natural abundance of each isotope varies in different regions of the
universe.
In the Tatooine region of space, the molar mass for a sample of chlorine is 36.20161 g/mol. Using only these three values, determine the relative percent abundance
of 35 Cl.
Enter the relative percent abundance of 35 Cl
76
Evaluate
Be careful with rounding too early. Each value is known to high precision, so your answer should reflect the one measurement of the group with lowest level of
precision.
Yellow Dye #5 Standard Curve
(?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning