Incorrect Your answer is incorrect. Suppose a 500. mL flask is filled with 0.90 mol of NO2, 1.4 mol of CO and 1.0 mol of NO. The following reaction becomes possible: NO2(g) +CO(g) = NO(g) +CO2(g) The equilibrium constant K for this reaction is 8.93 at the temperature of the flask. Calculate the equilibrium molarity of CO. Round your answer to two decimal places. 0.69 M ☑
Incorrect Your answer is incorrect. Suppose a 500. mL flask is filled with 0.90 mol of NO2, 1.4 mol of CO and 1.0 mol of NO. The following reaction becomes possible: NO2(g) +CO(g) = NO(g) +CO2(g) The equilibrium constant K for this reaction is 8.93 at the temperature of the flask. Calculate the equilibrium molarity of CO. Round your answer to two decimal places. 0.69 M ☑
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter14: Chemical Equilibirum
Section: Chapter Questions
Problem 14.13QP: A chemist put 1.18 mol of substance A and 2.85 mol of substance B into a 10.0-L flask, which she...
Related questions
Question
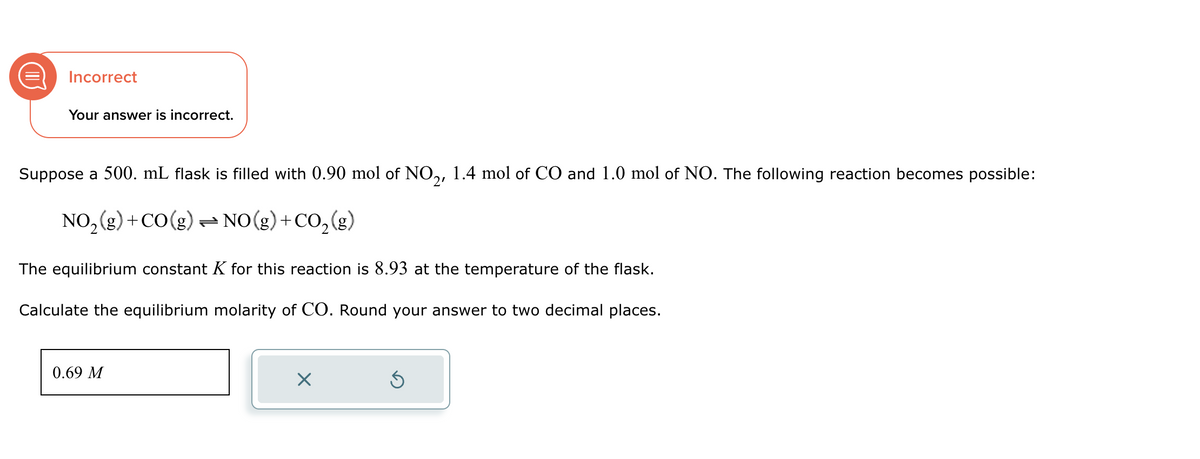
Transcribed Image Text:Incorrect
Your answer is incorrect.
Suppose a 500. mL flask is filled with 0.90 mol of NO2, 1.4 mol of CO and 1.0 mol of NO. The following reaction becomes possible:
NO2(g) +CO(g) = NO(g) +CO2(g)
The equilibrium constant K for this reaction is 8.93 at the temperature of the flask.
Calculate the equilibrium molarity of CO. Round your answer to two decimal places.
0.69 M
☑
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
