Indicate the intermolecular force (IMF) associated with each functional group: hydrogen-bonding, dipole-dipole, or dispersion / van der waals forces. Carboxylic acid Ester Arene Amide Alcohol Hydrogen Bonding Dipole-Dipole Dispersion Dipole-Dipole Hydrogen Bonding
Indicate the intermolecular force (IMF) associated with each functional group: hydrogen-bonding, dipole-dipole, or dispersion / van der waals forces. Carboxylic acid Ester Arene Amide Alcohol Hydrogen Bonding Dipole-Dipole Dispersion Dipole-Dipole Hydrogen Bonding
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter15: Aldehydes And Ketones
Section: Chapter Questions
Problem 15.4EP: In terms of polarity, which carbonyl group atom possesses a a. partial positive charge b. partial...
Related questions
Question
Indicate the intermolecular force (IMF) associated with each functional group: hydrogen-bonding, dipole-dipole, or dispersion / van der waals forces.

Transcribed Image Text:Indicate the intermolecular force (IMF) associated with each functional group:
hydrogen-bonding, dipole-dipole, or dispersion / van der waals forces.
Carboxylic acid
Ester
Arene
Amide
Alcohol
Hydrogen Bonding
Dipole-Dipole
Dispersion
Dipole-Dipole
Hydrogen Bonding
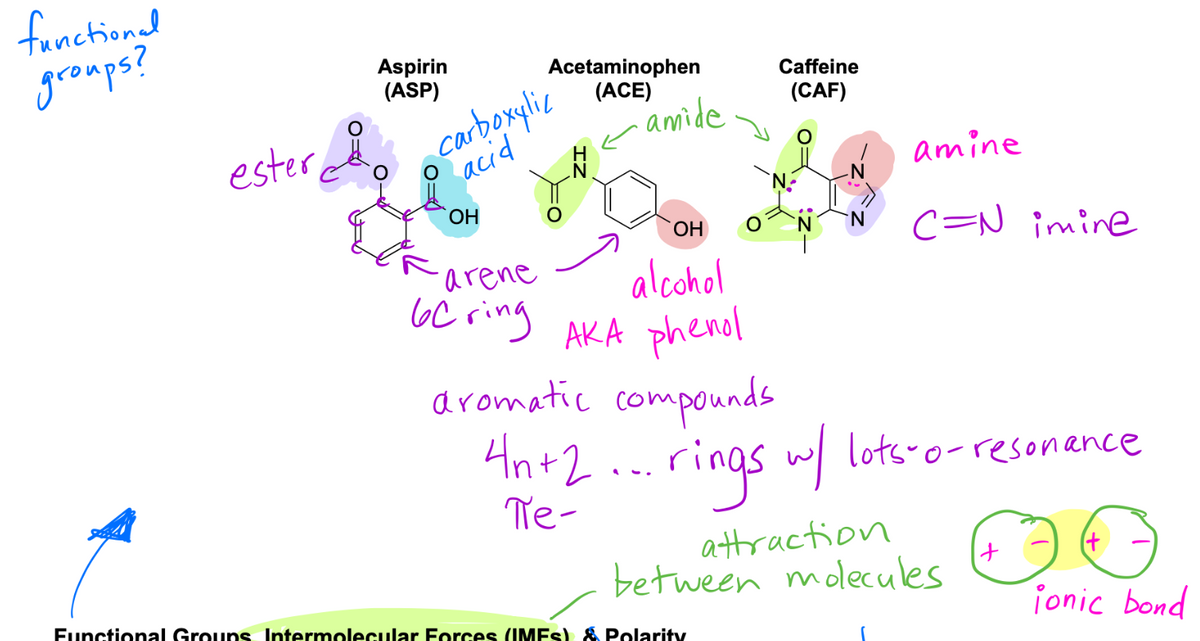
Transcribed Image Text:functional
groups?
Aspirin
(ASP)
ester co
Acetaminophen
(ACE)
。 carboxylic
o acid
OH
·amide
OH
·arene
alcohol
6C ring AKA phenol
aromatic compounds
4n+2
Te-
Caffeine
(CAF)
amine
C=N imine
rings w/ lots-o-resonance
attraction
Functional Groups Intermolecular Forces (IMFs) & Polarity
between molecules
+
(+
ionic bond
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning