Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 16QAP: Computers are not supposed to be in very warm rooms. The highest temperature tolerated for maximum...
Related questions
Question
Please do number 36.
(The answer is 3.09g. Please show me the work.)
Thank you!
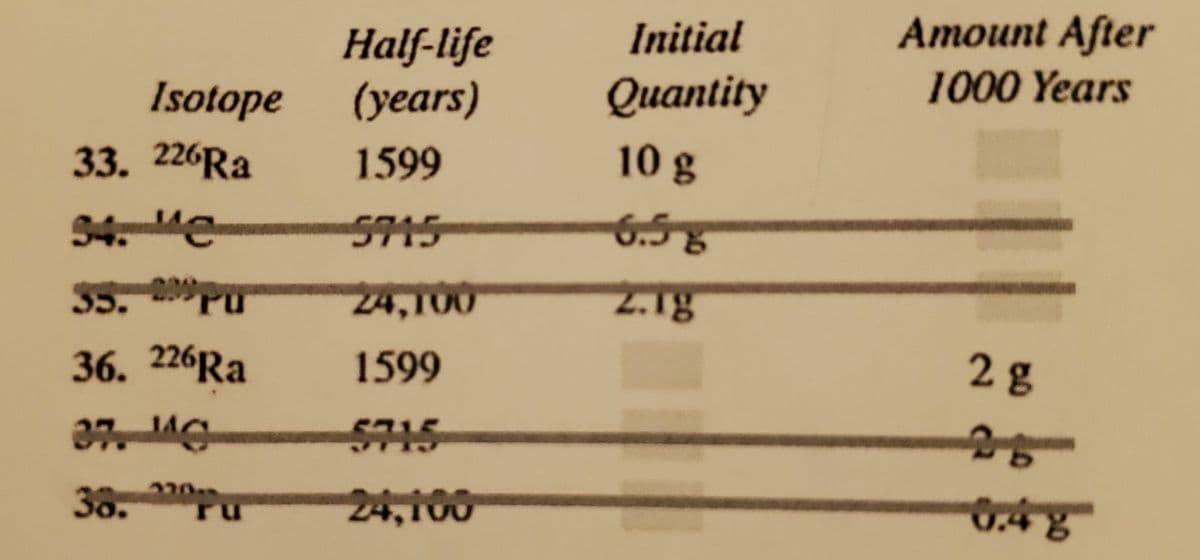
Transcribed Image Text:Initial
Amount After
Half-life
(years)
1000 Years
Isotope
Quantity
33. 226RA
1599
10g
94. e
5715
6.58
35.
Pu
24,100
2.1g
36. 226Ra
1599
28
2207
38.
24,100
0.48
ru
Expert Solution
Step 1 radioactivity
in radioactivity is defined as unstable nuclei is decay into stable nuclei and emits electromagnetic rays ,
Step 2 calculation
reaction between half life and= decay amount
A = A° (1/2)n
n = no. Of half life = t / t1/2
A° = initial amount
amount after 1000 years = 2g
t1/2 = 1599 years,
n = 1000/1599 = 0.625
2 = A° (1/2)0.625
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning