Radioactive Decay The half-life of palladium-100 is 4 days. After 20 days a sample has been reduced to a mass of 0.375 g. (a) What was the initial mass of the sample? (b) Find a function that models the mass remaining after 1 days. (c) What is the mass after 3 days? (d) After how many days will only 0.15 g remain?
Radioactive Decay The half-life of palladium-100 is 4 days. After 20 days a sample has been reduced to a mass of 0.375 g. (a) What was the initial mass of the sample? (b) Find a function that models the mass remaining after 1 days. (c) What is the mass after 3 days? (d) After how many days will only 0.15 g remain?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter18: Nuclear Chemistry
Section: Chapter Questions
Problem 71QRT
Related questions
Question
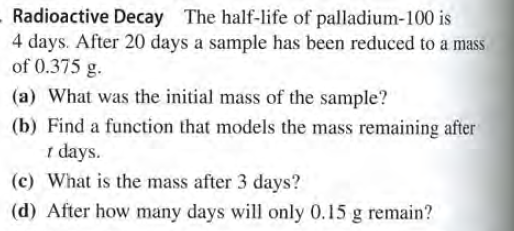
Transcribed Image Text:Radioactive Decay The half-life of palladium-100 is
4 days. After 20 days a sample has been reduced to a mass
of 0.375 g.
(a) What was the initial mass of the sample?
(b) Find a function that models the mass remaining after
1 days.
(c) What is the mass after 3 days?
(d) After how many days will only 0.15 g remain?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning