Instructions (questions 36–40): Consider the following reactions. Draw ALL POSSIBLE PRODUCTS, including stereochemistry and/or configuration whenever applicable. Write your answer in a piece of paper and then take a photo or scan your answer. Attach the answer. Br CH,0- 37. CH3 CH,OH 38. CHCH,CH3 Br CH3 39. HO" H- Br ČHĄCH3 CH3 40. CH,NH, CH;CH CI
Instructions (questions 36–40): Consider the following reactions. Draw ALL POSSIBLE PRODUCTS, including stereochemistry and/or configuration whenever applicable. Write your answer in a piece of paper and then take a photo or scan your answer. Attach the answer. Br CH,0- 37. CH3 CH,OH 38. CHCH,CH3 Br CH3 39. HO" H- Br ČHĄCH3 CH3 40. CH,NH, CH;CH CI
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter9: Nucleophilic Substitution And Β-elimination
Section: Chapter Questions
Problem 9.39P: Following are diastereomers (A) and (B) of 3-bromo-3,4-dimethylhexane. On treatment with sodium...
Related questions
Question
36 37
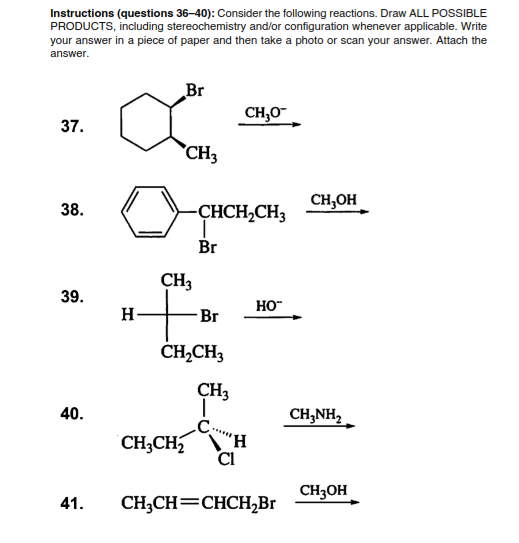
Transcribed Image Text:Instructions (questions 36–40): Consider the following reactions. Draw ALL POSSIBLE
PRODUCTS, including stereochemistry and/or configuration whenever applicable. Write
your answer in a piece of paper and then take a photo or scan your answer. Attach the
answer.
Br
CH,0-
37.
CH3
CH,OH
38.
CHCH,CH3
Br
CH3
39.
HO"
H-
Br
ČHĄCH3
CH3
40.
CH,NH,
H,
CI
CH3CH
CH3OH
41.
CH;CH=CHCH,Br
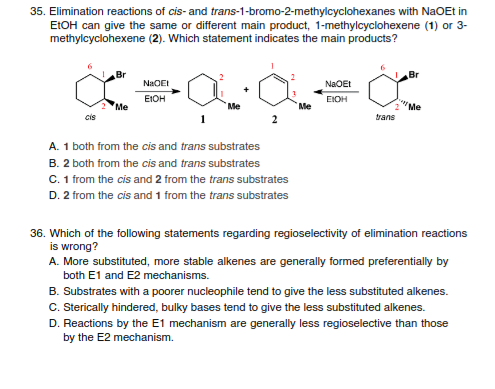
Transcribed Image Text:35. Elimination reactions of cis- and trans-1-bromo-2-methylcyclohexanes with NaOEt in
ELOH can give the same or different main product, 1-methylcyclohexene (1) or 3-
methylcyclohexene (2). Which statement indicates the main products?
Br
Br
NaOEI
NaOEt
EIOH
EIOH
Me
Me
Me
"Me
cis
trans
A. 1 both from the cis and trans substrates
B. 2 both from the cis and trans substrates
C. 1 from the cis and 2 from the trans substrates
D. 2 from the cis and 1 from the trans substrates
36. Which of the following statements regarding regioselectivity of elimination reactions
is wrong?
A. More substituted, more stable alkenes are generally formed preferentially by
both E1 and E2 mechanisms.
B. Substrates with a poorer nucleophile tend to give the less substituted alkenes.
C. Sterically hindered, bulky bases tend to give the less substituted alkenes.
D. Reactions by the E1 mechanism are generally less regioselective than those
by the E2 mechanism.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning