Interconversion of the staggered and eclipsed conformations of alkanes requires rotation around a -C bond such as the one depicted below (see arrow). Using your knowledge of bonding, explain why these rotations do not significantly affect the energy (strength) of these bonds. You may find it helpful to describe the type of bond being rotated.
Interconversion of the staggered and eclipsed conformations of alkanes requires rotation around a -C bond such as the one depicted below (see arrow). Using your knowledge of bonding, explain why these rotations do not significantly affect the energy (strength) of these bonds. You may find it helpful to describe the type of bond being rotated.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter10: Alcohols
Section: Chapter Questions
Problem 10.19P: Arrange these compounds in order of increasing boiling point (values in C are 42, 24, 78, and 118)....
Related questions
Question
Interconversion of the staggered and eclipsed conformations of alkanes
requires rotation around a -C bond such as the one depicted below (see arrow).
Using your knowledge of bonding, explain why these rotations do not significantly
affect the energy (strength) of these bonds. You may find it helpful to describe the
type of bond being rotated.
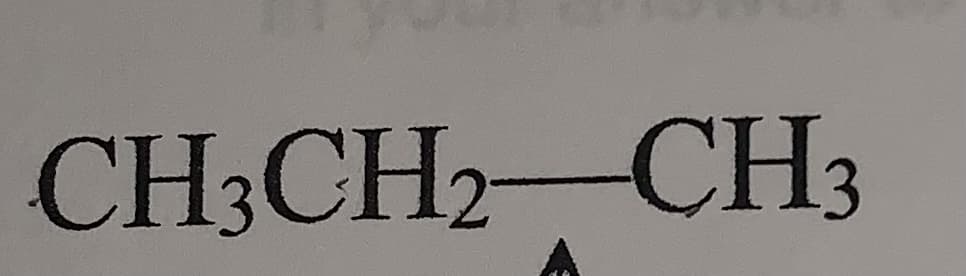
Transcribed Image Text:CH-CH—CHз
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning