Introduction Alkyl halides may undergo elimination reactions with Brønstead-Lowry bases in which the halide and an adjacent proton are lost to form a new π bond. Because of the loss of a proton and a halide anion the reactions are termed 'dehydrohalogenation'. The two most common associated mechanisms are designated as either unimolecular (E1) or bimolecular (E2) elimination reactions based on reaction rate studies. E1 reactions proceed via a two-step mechanism that involves the cleavage of the leaving group (here the halide) carbon bond to form a carbonium ion intermediate (slow step) that is followed by the transfer of a B-proton to a base (which can be quite weak and is usually the solvent) and the formation of the new π bond (fast step). Since the first step of the E1 is rate determining, the reaction is dependent only upon the concentration of the alkyl halide and not the concentration of the base. However, dehydrohalogentation reactions most commonly proceed via the E2 mechanism where the base abstracts a B-hydrogen atom at the same time as the halide ion is leaving to generate the new double bond. This one-step (concerted) mechanism proceeds through a single transition state and E2 reactions are termed 'bimolecular' because the rate at which they proceed is proportional to the multiplication product of the concentrations of both the alkyl halide and the base. Several factors must be considered to predict which elimination mechanism, E1 or E2, is likely a better description of the reaction. These are the strength of the base, concentration of attacking species, stability of the leaving group (halide anion), nature of the substrate, and solvent effects. This experiment is a case is an E2 elimination - in fact two of them. The action of potassium hydroxide in alcoholic solution on compounds containing halogen atoms on adjacent atoms (1,2-dihalides) results in the elimination of two molecules of hydrogen halide (double elimination) and the formation of two new π bonds to give a carbon-carbon triple bond. In this experiment you will perform a double elimination on the stilbene dibromide produced in the lab period two weeks previous (Experiment 5, Part 1) to synthesize the alkyne 1,2- diphenylacetylene according to the following reaction equation: H Br triethylene glycol + 2 KOH +2KBr + 2H₂O Br diphenylacetylene meso-1,2-dibromo-1,2-diphenylethane The final product will be characterized by TLC and UV-Vis spectroscopy. An overview of UV-Vis spectroscopy is provided in the textbook (9.2.4 in Ogilvie, 2018; 15.1-15.3 in Karty, 2014). The characteristic features of each compound in UV-Vis spectroscopy are the wavelength of maximum light absorbance (max) and the molar absorptivity or extinction coefficient ( or sometimes a), as given by the Beer-Lambert law (or Beer's Law): A = Ebc and A = absorbance (unitless), b (sometimes I) = path length (in cm), and c = concentration (in M). The units for & are therefore M-¹cm¹¹. CH
Introduction Alkyl halides may undergo elimination reactions with Brønstead-Lowry bases in which the halide and an adjacent proton are lost to form a new π bond. Because of the loss of a proton and a halide anion the reactions are termed 'dehydrohalogenation'. The two most common associated mechanisms are designated as either unimolecular (E1) or bimolecular (E2) elimination reactions based on reaction rate studies. E1 reactions proceed via a two-step mechanism that involves the cleavage of the leaving group (here the halide) carbon bond to form a carbonium ion intermediate (slow step) that is followed by the transfer of a B-proton to a base (which can be quite weak and is usually the solvent) and the formation of the new π bond (fast step). Since the first step of the E1 is rate determining, the reaction is dependent only upon the concentration of the alkyl halide and not the concentration of the base. However, dehydrohalogentation reactions most commonly proceed via the E2 mechanism where the base abstracts a B-hydrogen atom at the same time as the halide ion is leaving to generate the new double bond. This one-step (concerted) mechanism proceeds through a single transition state and E2 reactions are termed 'bimolecular' because the rate at which they proceed is proportional to the multiplication product of the concentrations of both the alkyl halide and the base. Several factors must be considered to predict which elimination mechanism, E1 or E2, is likely a better description of the reaction. These are the strength of the base, concentration of attacking species, stability of the leaving group (halide anion), nature of the substrate, and solvent effects. This experiment is a case is an E2 elimination - in fact two of them. The action of potassium hydroxide in alcoholic solution on compounds containing halogen atoms on adjacent atoms (1,2-dihalides) results in the elimination of two molecules of hydrogen halide (double elimination) and the formation of two new π bonds to give a carbon-carbon triple bond. In this experiment you will perform a double elimination on the stilbene dibromide produced in the lab period two weeks previous (Experiment 5, Part 1) to synthesize the alkyne 1,2- diphenylacetylene according to the following reaction equation: H Br triethylene glycol + 2 KOH +2KBr + 2H₂O Br diphenylacetylene meso-1,2-dibromo-1,2-diphenylethane The final product will be characterized by TLC and UV-Vis spectroscopy. An overview of UV-Vis spectroscopy is provided in the textbook (9.2.4 in Ogilvie, 2018; 15.1-15.3 in Karty, 2014). The characteristic features of each compound in UV-Vis spectroscopy are the wavelength of maximum light absorbance (max) and the molar absorptivity or extinction coefficient ( or sometimes a), as given by the Beer-Lambert law (or Beer's Law): A = Ebc and A = absorbance (unitless), b (sometimes I) = path length (in cm), and c = concentration (in M). The units for & are therefore M-¹cm¹¹. CH
Chapter14: Conjugated Compounds And Ultraviolet Spectroscopy
Section14.SE: Something Extra
Problem 23MP: Luminol, which is used by forensic scientists to find blood, fluoresces as a result of...
Related questions
Question
Create a table of physical properties and important SDS data for the reactants and product
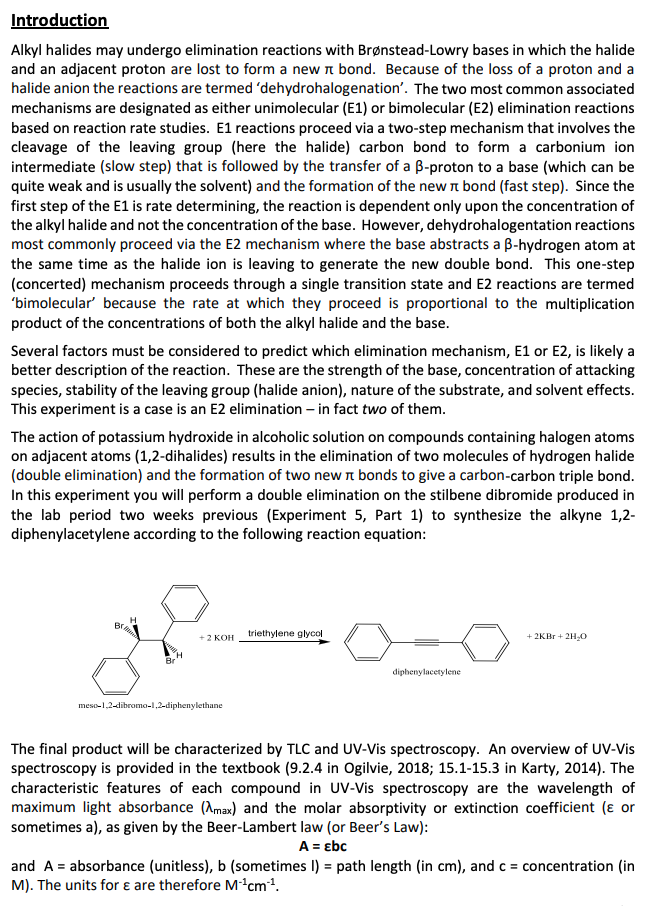
Transcribed Image Text:Introduction
Alkyl halides may undergo elimination reactions with Brønstead-Lowry bases in which the halide
and an adjacent proton are lost to form a new π bond. Because of the loss of a proton and a
halide anion the reactions are termed 'dehydrohalogenation'. The two most common associated
mechanisms are designated as either unimolecular (E1) or bimolecular (E2) elimination reactions
based on reaction rate studies. E1 reactions proceed via a two-step mechanism that involves the
cleavage of the leaving group (here the halide) carbon bond to form a carbonium ion
intermediate (slow step) that is followed by the transfer of a B-proton to a base (which can be
quite weak and is usually the solvent) and the formation of the new π bond (fast step). Since the
first step of the E1 is rate determining, the reaction is dependent only upon the concentration of
the alkyl halide and not the concentration of the base. However, dehydrohalogentation reactions
most commonly proceed via the E2 mechanism where the base abstracts a B-hydrogen atom at
the same time as the halide ion is leaving to generate the new double bond. This one-step
(concerted) mechanism proceeds through a single transition state and E2 reactions are termed
'bimolecular' because the rate at which they proceed is proportional to the multiplication
product of the concentrations of both the alkyl halide and the base.
Several factors must be considered to predict which elimination mechanism, E1 or E2, is likely a
better description of the reaction. These are the strength of the base, concentration of attacking
species, stability of the leaving group (halide anion), nature of the substrate, and solvent effects.
This experiment is a case is an E2 elimination - in fact two of them.
The action of potassium hydroxide in alcoholic solution on compounds containing halogen atoms
on adjacent atoms (1,2-dihalides) results in the elimination of two molecules of hydrogen halide
(double elimination) and the formation of two new π bonds to give a carbon-carbon triple bond.
In this experiment you will perform a double elimination on the stilbene dibromide produced in
the lab period two weeks previous (Experiment 5, Part 1) to synthesize the alkyne 1,2-
diphenylacetylene according to the following reaction equation:
H
Br
triethylene glycol
+ 2 KOH
+2KBr + 2H₂O
Br
diphenylacetylene
meso-1,2-dibromo-1,2-diphenylethane
The final product will be characterized by TLC and UV-Vis spectroscopy. An overview of UV-Vis
spectroscopy is provided in the textbook (9.2.4 in Ogilvie, 2018; 15.1-15.3 in Karty, 2014). The
characteristic features of each compound in UV-Vis spectroscopy are the wavelength of
maximum light absorbance (max) and the molar absorptivity or extinction coefficient ( or
sometimes a), as given by the Beer-Lambert law (or Beer's Law):
A = Ebc
and A = absorbance (unitless), b (sometimes I) = path length (in cm), and c = concentration (in
M). The units for e are therefore M-¹cm¹.
CH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

