Introduction to Polymers Some Applications of Polymers Properties of Polymers Introduction to Polymers Polymer is a large molecule composed of repeating small molecule units. The was derived from the Greek words poly and meros, which means many and parts, re often associate polymers with the term plastics. In reality, these plastics are just som synthetic polymers commonly encountered and used daily such as grocery bags and w The small molecule precursor or building block of polymers is called a monomer polyethylene which is commonly used in pipes, cable coverings, and toys is derived fro 7.1a). Often, the chemical structure of polymers is represented by the monomer parenthesis (Fig 7.1b). The number of repeating monomer units is represented by the s Monomer Polymer H. H нн C=C -С-с- H нн H H/n Ethene Polyethylene (b) (a) Figure 7.1. Monomer ethene (a) and chemical structure of polyethylene (b). Some Applications of Polymers
Introduction to Polymers Some Applications of Polymers Properties of Polymers Introduction to Polymers Polymer is a large molecule composed of repeating small molecule units. The was derived from the Greek words poly and meros, which means many and parts, re often associate polymers with the term plastics. In reality, these plastics are just som synthetic polymers commonly encountered and used daily such as grocery bags and w The small molecule precursor or building block of polymers is called a monomer polyethylene which is commonly used in pipes, cable coverings, and toys is derived fro 7.1a). Often, the chemical structure of polymers is represented by the monomer parenthesis (Fig 7.1b). The number of repeating monomer units is represented by the s Monomer Polymer H. H нн C=C -С-с- H нн H H/n Ethene Polyethylene (b) (a) Figure 7.1. Monomer ethene (a) and chemical structure of polyethylene (b). Some Applications of Polymers
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter23: Carbon: Not Just Another Element
Section: Chapter Questions
Problem 120SCQ
Related questions
Question
Note: I need a simple explanation for this lesson for my presentation. Thank you!
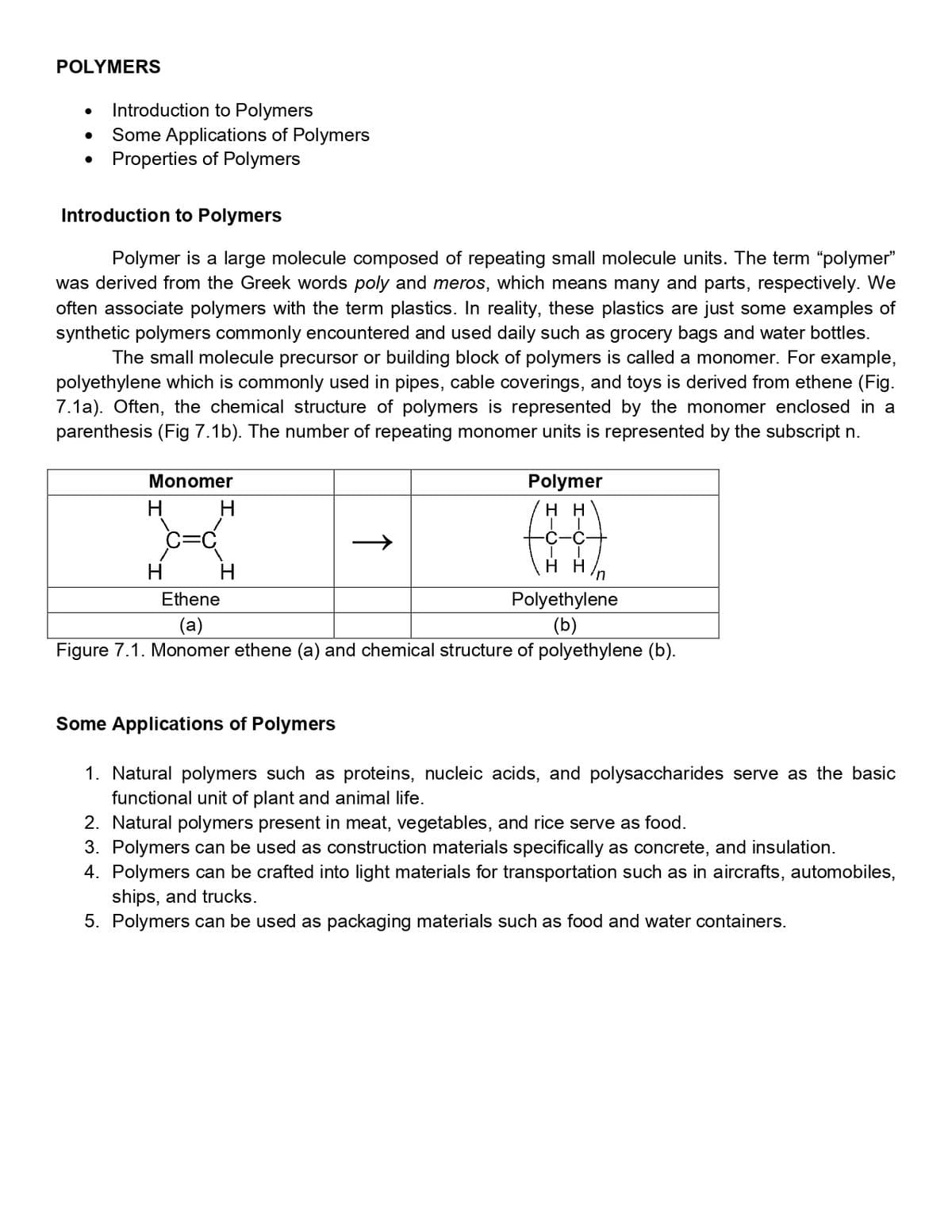
Transcribed Image Text:POLYMERS
Introduction to Polymers
Some Applications of Polymers
• Properties of Polymers
Introduction to Polymers
Polymer is a large molecule composed of repeating small molecule units. The term "polymer"
was derived from the Greek words poly and meros, which means many and parts, respectively. We
often associate polymers with the term plastics. In reality, these plastics are just some examples of
synthetic polymers commonly encountered and used daily such as grocery bags and water bottles.
The small molecule precursor or building block of polymers is called a monomer. For example,
polyethylene which is commonly used in pipes, cable coverings, and toys is derived from ethene (Fig.
7.1a). Often, the chemical structure of polymers is represented by the monomer enclosed in a
parenthesis (Fig 7.1b). The number of repeating monomer units is represented by the subscript n.
Monomer
Polymer
H
н
H
H
н
un
Ethene
Polyethylene
(a)
(b)
Figure 7.1. Monomer ethene (a) and chemical structure of polyethylene (b).
Some Applications of Polymers
1. Natural polymers such as proteins, nucleic acids, and polysaccharides serve as the basic
functional unit of plant and animal life.
2. Natural polymers present in meat, vegetables, and rice serve as food.
3. Polymers can be used as construction materials specifically as concrete, and insulation.
4. Polymers can be crafted into light materials for transportation such as in aircrafts, automobiles,
ships, and trucks.
5. Polymers can be used as packaging materials such as food and water containers.
↑
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning