is desired to separate silver in a solution that is 0.0613 M 0.0955 Ag', and 0.500 by controlled-potential electrolysis. Ag*(aq) + 1 e¯ –→ Ag(s) E° = 0.799 V Cu²+ + 2e¯ Cu(s) E° = 0.337 V Bio* (aq) + 2 H*(aq) + 3 e¯ → Bi(s) + H,O(1) E° = 0.320 V Between what cell potentials can silver be quantitatively removed from the solution without also removing one of the othe ions using controlled-potential electrolysis? Use 1.00 x 10-6 M as the criterion for quantitative removal. Report all cell potentials versus Ag|AgCl (E = 0.197 V). List the higher (more positive) potential first. between Ecell = V and Ecell = V
is desired to separate silver in a solution that is 0.0613 M 0.0955 Ag', and 0.500 by controlled-potential electrolysis. Ag*(aq) + 1 e¯ –→ Ag(s) E° = 0.799 V Cu²+ + 2e¯ Cu(s) E° = 0.337 V Bio* (aq) + 2 H*(aq) + 3 e¯ → Bi(s) + H,O(1) E° = 0.320 V Between what cell potentials can silver be quantitatively removed from the solution without also removing one of the othe ions using controlled-potential electrolysis? Use 1.00 x 10-6 M as the criterion for quantitative removal. Report all cell potentials versus Ag|AgCl (E = 0.197 V). List the higher (more positive) potential first. between Ecell = V and Ecell = V
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter24: Coulometry
Section: Chapter Questions
Problem 24.4QAP: Halide ions can he deposited at a silver anode, the reaction being Ag(s) + X- AgX(s) +e- Suppose...
Related questions
Question
It is desired to separate silver in a solution that is 0.06130.0613 M BiO+BiO+, 0.1290.129 M Cu2+Cu2+, 0.09550.0955 M Ag+Ag+, and 0.5000.500 M HClO4HClO4 by controlled-potential electrolysis
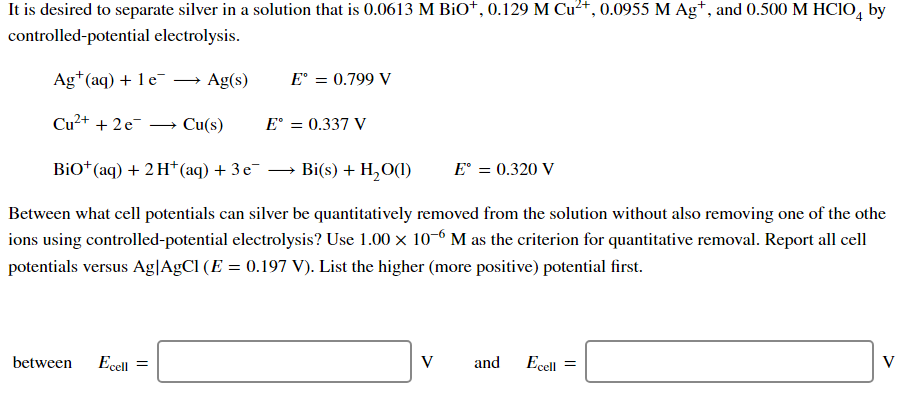
Transcribed Image Text:It is desired to separate silver in a solution that is 0.0613 M BiO*, 0.129 M Cu²+, 0.0955 M Ag*, and 0.500 M HCIO, by
controlled-potential electrolysis.
Ag*(aq) + 1 e-
Ag(s)
E° = 0.799 V
Cu²+ + 2e
Cu(s)
E° = 0.337 V
BiO*(aq) + 2 H* (aq) + 3 e¯ → Bi(s) + H,O(1)
E° = 0.320 V
Between what cell potentials can silver be quantitatively removed from the solution without also removing one of the othe
ions using controlled-potential electrolysis? Use 1.00 x 10-6 M as the criterion for quantitative removal. Report all cell
potentials versus Ag|AgCl (E = 0.197 V). List the higher (more positive) potential first.
between
Ecell =
V
and
Ecell =
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning