is polar 1. The main reason why CH3 OH has a higher vapor pressure at a given temperature when does not exhibit dispersion compared to CH3CH2CH2OH is that CH3 OH forces 2. The main reasons why CH4 has a higher vapor pressure at a given temperature when compared does not exhibit hydrogen bonding to CH3C1 is that CH4 and has a smaller molar mass 3. The main reasons why H2 CO has a higher vapor pressure at a given temperature when does not exhibit dipole-dipole compared to CH3OH is that H2CO and forces
is polar 1. The main reason why CH3 OH has a higher vapor pressure at a given temperature when does not exhibit dispersion compared to CH3CH2CH2OH is that CH3 OH forces 2. The main reasons why CH4 has a higher vapor pressure at a given temperature when compared does not exhibit hydrogen bonding to CH3C1 is that CH4 and has a smaller molar mass 3. The main reasons why H2 CO has a higher vapor pressure at a given temperature when does not exhibit dipole-dipole compared to CH3OH is that H2CO and forces
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 93CP: Which of the following compound(s) exhibit only London dispersion intermolecular forces? Which...
Related questions
Question
Transcribed Image Text:Reset
Help
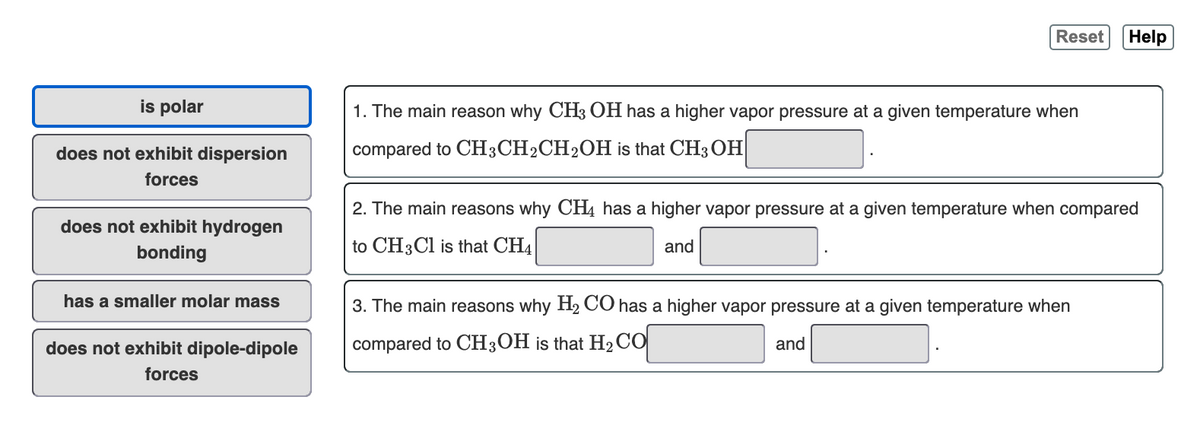
is polar
1. The main reason why CH3 OH has a higher vapor pressure at a given temperature when
does not exhibit dispersion
compared to CH3CH2CH2OH is that CH3 OH
forces
2. The main reasons why CH4 has a higher vapor pressure at a given temperature when compared
does not exhibit hydrogen
to CH3CI is that CH4
and
bonding
has a smaller molar mass
3. The main reasons why H2 CO has a higher vapor pressure at a given temperature when
does not exhibit dipole-dipole
compared to CH3OH is that H2CO
and
forces
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning