Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy AH per mole of KOH. rxn
Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy AH per mole of KOH. rxn
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 42P
Related questions
Question
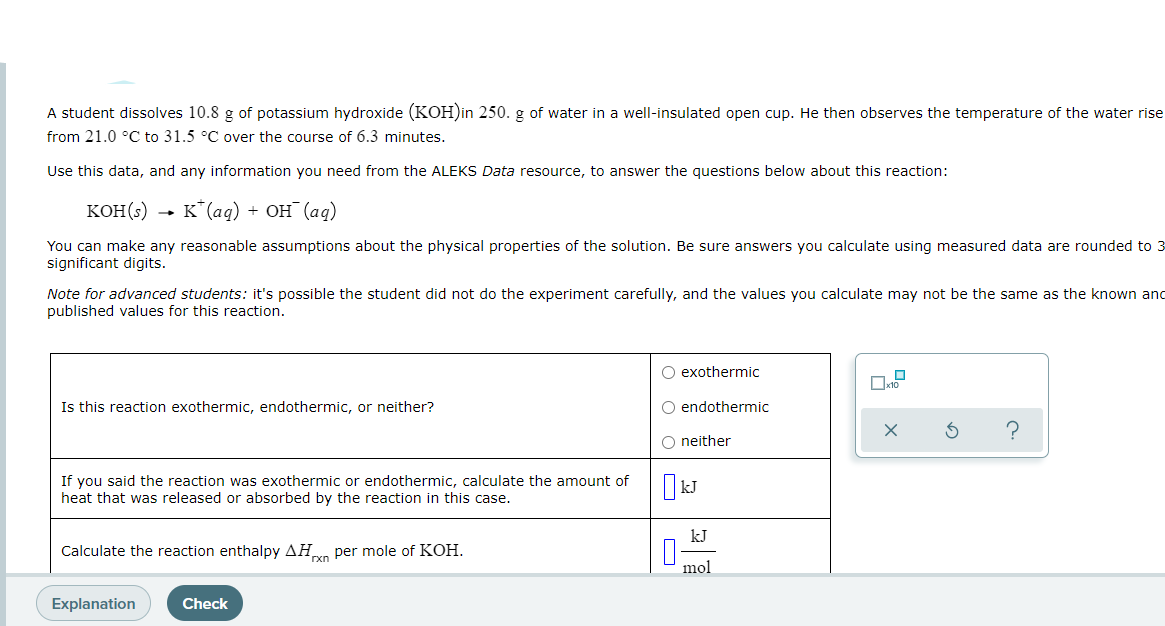
Transcribed Image Text:A student dissolves 10.8 g of potassium hydroxide (KOH)in 250. g of water in a well-insulated open cup. He then observes the temperature of the water rise
from 21.0 °C to 31.5 °C over the course of 6.3 minutes.
Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction:
коН(б) — к*(ад) + он (аg)
You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 3
significant digits.
Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and
published values for this reaction.
O exothermic
Is this reaction exothermic, endothermic, or neither?
O endothermic
O neither
If you said the reaction was exothermic or endothermic, calculate the amount of
heat that was released or absorbed by the reaction in this case.
kJ
Calculate the reaction enthalpy AHn per mole of KOH.
rxn
mol
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning