Isooctane is burned with theoretical air with 20% excess air during the combustion process in a small 3 – cylinder turbocharged automobile engine. Calculate: 1. Air – fuel ratio 2. Fuel – air ration 3. Equivalence ratio Stoichiometric reaction: _C3H8 + 02 + N2 → _CO2+ _H,0 + N2 With 20% excess air: _C3H8 + 02 + N2 →, CO2 + _H20 +. N2
Isooctane is burned with theoretical air with 20% excess air during the combustion process in a small 3 – cylinder turbocharged automobile engine. Calculate: 1. Air – fuel ratio 2. Fuel – air ration 3. Equivalence ratio Stoichiometric reaction: _C3H8 + 02 + N2 → _CO2+ _H,0 + N2 With 20% excess air: _C3H8 + 02 + N2 →, CO2 + _H20 +. N2
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter32: Oil Heat
Section: Chapter Questions
Problem 12RQ: When incomplete combustion occurs, what additional by-products of combustion are created?
Related questions
Question
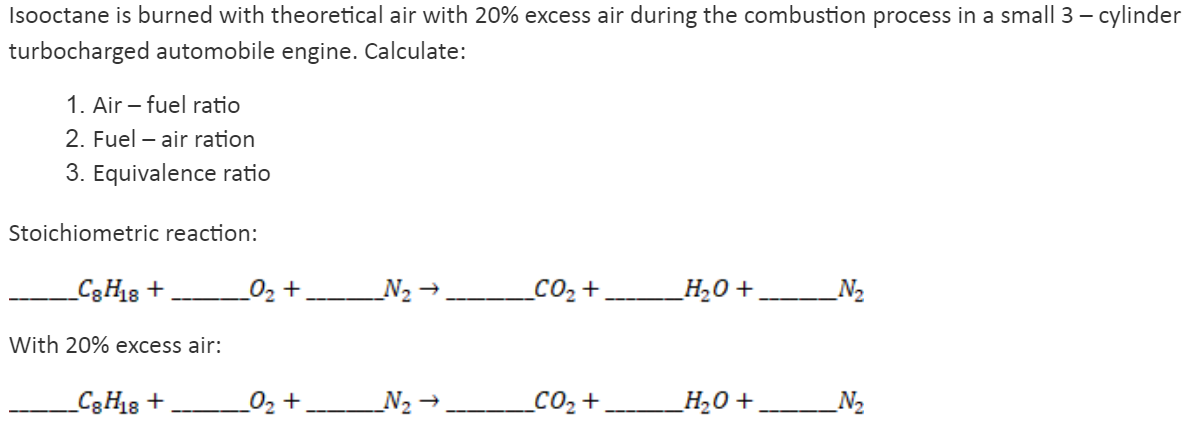
Transcribed Image Text:Isooctane is burned with theoretical air with 20% excess air during the combustion process in a small 3 – cylinder
turbocharged automobile engine. Calculate:
1. Air – fuel ratio
2. Fuel – air ration
3. Equivalence ratio
Stoichiometric reaction:
_C3H8 +
02 +
N2 →
_CO2+
_H,0 + N2
With 20% excess air:
_C3H8 +
02 + N2 →,
CO2 +
_H20 +.
N2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning