It can be shown that the maximum in the wavelength distribution for blackbody radiation can be approximated well by the formula hc AmaxT= 4.965 kB where h = 6.62607015 x 10-34 J.sec, kg = 1.38068 x 10-23 J/K, and c 2.99792458 x 108 m/sec, T is in K, and Amax is given in m. (b) If the visible portion of the electromagnetic spectrum spans the wavelengths from 400 to 700 nm, what is the smallest temperature that would have its value of Amax in the visible range? Report your answer in K.
It can be shown that the maximum in the wavelength distribution for blackbody radiation can be approximated well by the formula hc AmaxT= 4.965 kB where h = 6.62607015 x 10-34 J.sec, kg = 1.38068 x 10-23 J/K, and c 2.99792458 x 108 m/sec, T is in K, and Amax is given in m. (b) If the visible portion of the electromagnetic spectrum spans the wavelengths from 400 to 700 nm, what is the smallest temperature that would have its value of Amax in the visible range? Report your answer in K.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 26QRT
Related questions
Question
Solve only Q12 in 30 minute
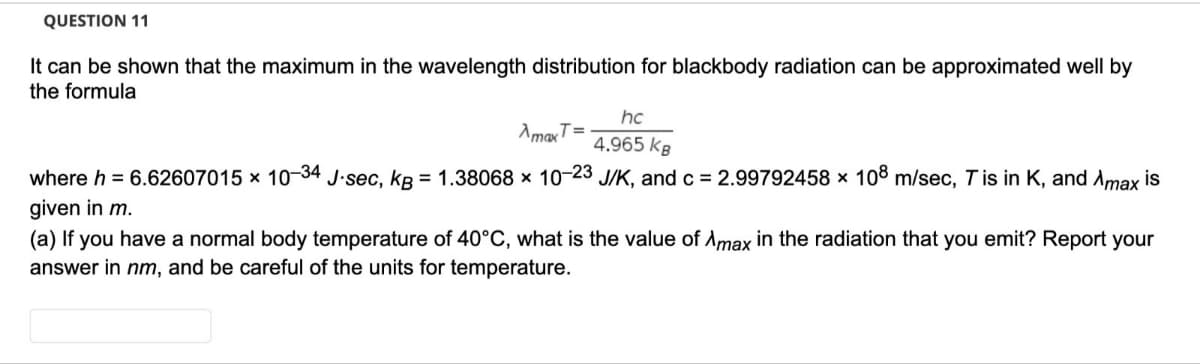
Transcribed Image Text:QUESTION 11
It can be shown that the maximum in the wavelength distribution for blackbody radiation can be approximated well by
the formula
hc
AmaxT=
4.965 kg
where h = 6.62607015 × 10-34 J.sec, kB = 1.38068 × 10-23 J/K, and c = 2.99792458 × 108 m/sec, Tis in K, and Amax is
given in m.
(a) If you have a normal body temperature of 40°C, what is the value of Amax in the radiation that you emit? Report your
answer in nm, and be careful of the units for temperature.
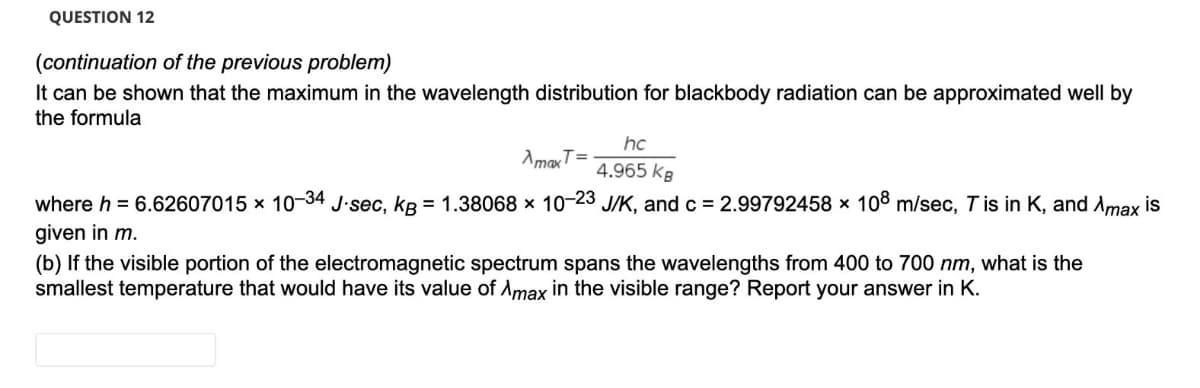
Transcribed Image Text:QUESTION 12
(continuation of the previous problem)
It can be shown that the maximum in the wavelength distribution for blackbody radiation can be approximated well by
the formula
hc
AmaxT=
4.965 kB
where h = 6.62607015 x 10-34 J-sec, kB = 1.38068 x 10-23 J/K, and c = 2.99792458 x 108 m/sec, Tis in K, and Amax is
given in m.
(b) If the visible portion of the electromagnetic spectrum spans the wavelengths from 400 to 700 nm, what is the
smallest temperature that would have its value of Amax in the visible range? Report your answer in K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning